Invented by Emma Masteller, Thomas Brennan, David Bellovin, Kevin P. Baker, Brian Wong, Five Prime Therapeutics Inc
Combination therapy has emerged as a promising approach in the field of cancer treatment, with the potential to enhance the effectiveness of existing therapies and improve patient outcomes. One such combination therapy that has gained significant attention is the use of anti-CSF1R and anti-CD40 antibodies for cancer treatment. This article will explore the market for this combination therapy and its potential impact on the field of oncology.
CSF1R (colony-stimulating factor 1 receptor) and CD40 are both immune checkpoint proteins that play crucial roles in regulating the immune response. CSF1R is involved in the recruitment and activation of tumor-associated macrophages (TAMs), which can promote tumor growth and suppress the immune system. CD40, on the other hand, is expressed on antigen-presenting cells and is involved in the activation of T cells and the production of pro-inflammatory cytokines.
By targeting both CSF1R and CD40 simultaneously, researchers hope to achieve a synergistic effect that can enhance the immune response against cancer cells. Anti-CSF1R antibodies can deplete TAMs, thereby reducing the immunosuppressive tumor microenvironment. Anti-CD40 antibodies can activate antigen-presenting cells and promote T cell-mediated anti-tumor responses. Together, these antibodies have the potential to unleash the full power of the immune system against cancer.
The market for combination therapy of anti-CSF1R and anti-CD40 antibodies is still in its early stages, with several ongoing clinical trials investigating the safety and efficacy of this approach. However, the results so far have been promising. In a phase 1b clinical trial, the combination of anti-CSF1R and anti-CD40 antibodies showed encouraging anti-tumor activity in patients with advanced solid tumors. This has generated significant interest from both pharmaceutical companies and investors, leading to a surge in research and development activities in this area.
Several major pharmaceutical companies have recognized the potential of this combination therapy and have initiated collaborations and partnerships to develop novel anti-CSF1R and anti-CD40 antibody therapies. These companies are investing heavily in research and development, clinical trials, and manufacturing capabilities to bring these therapies to market. The market for combination therapy of anti-CSF1R and anti-CD40 antibodies is expected to witness substantial growth in the coming years as more clinical data becomes available and regulatory approvals are obtained.
The potential market for this combination therapy is vast, considering the prevalence of cancer worldwide. According to the World Health Organization (WHO), cancer is the second leading cause of death globally, with an estimated 9.6 million deaths in 2018. The demand for effective and innovative cancer therapies is high, and combination therapies like anti-CSF1R and anti-CD40 antibodies hold great promise in addressing this unmet medical need.
In conclusion, the market for combination therapy of anti-CSF1R and anti-CD40 antibodies for cancer is a rapidly growing field with immense potential. The synergistic effect of targeting both CSF1R and CD40 has shown promising results in early clinical trials, leading to increased investment and research in this area. As more data becomes available, and regulatory approvals are obtained, this combination therapy has the potential to revolutionize cancer treatment and improve patient outcomes.

The Five Prime Therapeutics Inc invention works as follows
The combination of one or more immune-stimulating agents with antibodies that bind to colony stimulating factor receptor (CSF1R), in order to treat cancer, is described.

Background for Combination therapy of anti-CSF1R and anti-CD40 antibodies for cancer
The “Colony Stimulating Factor 1 Receptor” (also referred to as CSF1R, also known in the art by FMS, FIM2, CFMS, M CSF receptor and CD115) consists of a single-pass, transmembrane, receptor with an extracellular domain at its N-terminus and a C terminal intracellular domain that has tyrosine-kinase activities. CSF1 and the interleukin 34 ligand, (referred to as IL34 herein; Lin et. al., Science 320, 807-11 (2008)), bind to CSF1R, causing receptor dimerization. This leads to an increase in CSF1R protein tyrosine kinase activities, phosphorylation at CSF1R tyrosine residua, and downstream signaling. CSF1R activation leads to the survival, proliferation and differentiation of macrophages and monocytes as well as other monocytic cells such as osteoclasts.
Many tumor cells and tumor stromal cell have been shown to produce CSF1, activating monocyte/macrophages cells via CSF1R. It has been demonstrated that the amount of CSF1 found in tumors correlates with the number of tumor-associated macacaphages. In most cancers, higher levels of TAMs correlate with a worse prognosis. CSF1 was also found to promote tumor progression and growth in human breast cancer mouse xenografts. See, e.g., Paulus et al., Cancer Res. 66: 4349-56 (2006). CSF1R also plays a part in osteolytic bone degradation in bone metastasis. See, e.g., Ohno et al., Mol. Cancer Ther. 5: 2634-43 (2006). TAMs can promote tumor growth in part by suppressing antitumor T-cell effector function via the release of immunesuppressive cytokines or the expression of T cell inhibiting surface proteins. “Antibodies that bind CSF1R could be used in cancer treatment.
Some tumor cells can escape detection by immune system in at least part by suppressing immune response. For example, by altering expression of immune modulatory gene. The relative concentrations of immune inhibitory and stimulatory molecules in the body, for example, can modulate the adaptive immunity response. The expression of inhibitory molecule and/or certain stimulatory molecule at a relatively high level or reduced expression may act as a switch to down-regulate the adaptive immune system. Potential cancer therapies are agents that counteract this by up-regulating adaptive immune responses or by stimulating the immune system.
A combination regimen of agents modulating the immune response to cancer, such immune stimulating agents, can increase the depth and duration of the response, and also provide a wider range of efficacy for patients who don’t respond to one agent alone.
In some embodiments, cancer treatment methods are provided that include administering an anti-CSF1R antigen and at least one immunostimulating agent to a subject. In some embodiments the immune stimulating agents are an agonist or co-stimulatory molecules of immune-stimulatory molecules, whereas in other embodiments the immune stimulating agents are antagonists of immune inhibitory molecules, including co-inhibitory molecules. In some embodiments the at least one immuno stimulating agent is an agonist, which can be found on immune cells such as T-cells. In some embodiments the at least one immuno stimulating agent is an antagonist of an anti-inhibitory molecular, including a coinhibitory molecular, found on immune cell, such as T-cells. In some embodiments the at least one immuno stimulating agent is an agonist of a immune-stimulatory molecule. This includes a costimulatory molecule found on cells that are involved in innate immune system, such as NK-cells. In some embodiments the immune stimulating agent is an antagonist of an immuno-inhibitory molecule. This includes a coinhibitory molecule found on cells that are involved in innate immune, such as NK-cells. In some embodiments, a combination of the two agents enhances either the antigen-specific response by T cells in the treated subjects or the innate immune response. In some embodiments the combination leads to an improved antitumor response when administered in an animal cancer models, such as the xenograft, than either the anti CSF1R antibody alone or the immune stimulating agent. In some embodiments the combination produces a synergistic effect in an animal cancer models, such as the xenograft, when compared with administration of the anti-CSF1R antibodies or immune stimulating agents alone.
In some embodiments, the at least one immune stimulating agent comprises an antagonist of an inhibitor of the activation of T cells, while in some embodiments, the at least one immune stimulating agent comprises comprises an agonist of a stimulator of the activation of T cells. In some embodiments, the at least one immune stimulating agent comprises an antagonist of CTLA4, LAG-3, Galectin 1, Galectin 9, CEACAM-1, BTLA, CD25, CD69, TIGIT, CD113, GPR56, VISTA, B7-H3, B7-H4, 2B4, CD48, GARP, PD1H, LAIR1, TIM1, TIM3, TIM4, ILT4, IL-6, IL-10, TGF?, VEGF, KIR, LAG-3, adenosine A2A receptor, PI3Kdelta, or IDO. In some embodiments, the at least one immune stimulating agent comprises an agonist of B7-1, B7-2, CD28, 4-1BB (CD137), 4-1BBL, ICOS, ICOS-L, OX40, OX40L, GITR, GITRL, CD27, CD40, CD40L, DR3, CD28H, IL-2, IL-7, IL-12, IL-15, IL-21, IFN?, STING or a Toll-like receptor agonist such as a TLR2/4 agonist. In some embodiments, the at least one immune stimulating agent comprises an agent that binds to a member of the B7 family of membrane-bound proteins such as B7-1, B7-2, B7-H2 (ICOS-L), B7-H3, B7-H4, B7-H5 (VISTA), and B7-H6. In some embodiments, the at least one immune stimulating agent comprises an agent that binds to a member of the TNF receptor family or a co-stimulatory or co-inhibitory molecule binding to a member of the TNF receptor family such as CD40, CD40L, OX40, OX40L, GITR, GITRL, CD70, CD27L, CD30, CD30L, 4-1BBL, CD137 (4-1BB), TRAIL/Apo2-L, TRAILR1/DR4, TRAILR2/DR5, TRAILR3, TRAILR4, OPG, RANK, RANKL, TWEAKR/Fn14, TWEAK, BAFFR, EDAR, XEDAR, EDA1, EDA2, TACI, APRIL, BCMA, LT?R, LIGHT, DeR3, HVEM, VEGL/TL1A, TRAMP/DR3, TNFR1, TNF?, TNFR2, TNF?, 1?2, FAS, FASL, RELT, DR6, TROY, or NGF?. In some embodiments, the at least one immune stimulating agent comprises an agent that antagonizes or inhibits a cytokine that inhibits T cell activation such as IL-6, IL-10, TGF?, VEGF. In some embodiments, the at least one immune stimulating agent comprises an antagonist of a chemokine, such as CXCR2, CXCR4, CCR2, or CCR4. In some embodiments, the at least one immune stimulating agent comprises an agonist of a cytokine that stimulates T cell activation such as IL-2, IL-7, IL-12, IL-15, IL-21, and IFN?. In some embodiments, the at least one immune stimulating agent comprises an antibody. In some embodiments, the at least one immune stimulating agent may comprise a vaccine, such as a mesothelin-targeting vaccine or attenuated listeria cancer vaccine such as CRS-207. Any one or more of the above antagonists, agonists, and binding agents may be combined with any one or more of the anti-CSF1R antibodies described herein.
In some embodiments, at least one immunostimulating agent is a CD40-agonist, which can be used in combination with one or more of the other immune stimulators listed above. In some embodiments the CD40-agonist is an antigen. In some embodiments the CD40 antagonist is an anti CD40 antibody. In some embodiments the anti-CD40 antibodies are the CDRs from an antibody selected among CP-870.893, dacetuzumab, SEA-CD40, ADC-1013, RO7009789, and Chi Lob 7.4 In some embodiments the anti-CD40 antibodies comprises the heavy and light chain variable region of an antibody chosen from CP-870.893, dacetuzumab, SEA-CD40, ADC-1013, RO7009789, and Chi Lob 7.4 In some embodiments the anti-CD40 antibodies are selected from CP-870.893, dacetuzumab, SEA-CD40, ADC-1013, RO7009789, and Chi Lob 7.4. In some embodiments the CD40 agonist can be recombinant CD40L. In some embodiments the at least immune stimulating agents comprise a CD40-agonist and at most one additional immune stimulant from those described above. “For example, any of the above immunostimulating agents may be combined with one or more anti-CSF1R antibody described herein, as well as a CD40 antagonist, such a CD40-agonist antibody, or recombinant CD40L such as any of the antiCD40 antibodies described previously.
In some embodiments the anti-CSF1R antibodies and at least one immunostimulatory agent may be administered simultaneously or sequentially. In some embodiments the anti-CSF1R and at least one immunostimulatory agent are given concurrently. In some embodiments one or more doses are given of the immune stimulatory drug before administering anti-CSF1R antibodies. In some embodiments the subject has received the complete course of treatment with the immune stimulatory agents before the administration of the CSF1R antibodies. In some embodiments the anti-CSF1R is administered as part of a second course with at least one immunostimulatory agent. In some embodiments the subject has received atleast one, two, three or four doses of atleast one immune stimulating agent before the anti-CSF1R antibodies. In some embodiments at least one dose is given concurrently with anti-CSF1R inhibition. In some embodiments one or more anti-CSF1R antibodies are administered before administering the immune stimulatory agents. In some embodiments the subject receives at least two doses of anti-CSF1R before receiving at least one immunostimulatory agent. In some embodiments at least one anti-CSF1R dose is administered simultaneously with at least one immunostimulatory agent.
In some embodiments, cancers include non-small cell lung carcinoma, melanoma (squamous cells of the head and throat), ovarian, kidney, liver, colon, breast, skin, skin cancer and uterine cancer. In some embodiments the cancer is recurrent after treatment. This can be surgery, chemotherapy or radiation therapy.
In some embodiments, there are compositions that include an anti-CSF1R antibodies and at least one immunostimulatory agent. In some embodiments the immune stimulating agents are antagonists of inhibitors of activation T cells. In other embodiments the immune stimulating agents are agonists In some embodiments the immune stimulating agent is an antagonist for CTLA4, LAG-3 (CD137), Galectin 1, Galectin 9 CEA In some embodiments the immune stimulating agent is an agonist for B7-1, -2, 4-1BB, OX40L (CD137), In some embodiments the immune stimulating agent is an agent that binds a membrane-bound protein of the B7 subfamily, such as B7 In some embodiments the immune stimulating agent is an agent that binds a TNF receptor or a costimulatory or a coin In some embodiments the at least one immunostimulating agent is an agent that antagonizes a cytokine inhibiting T cell In some embodiments the at least one immunostimulating agent is an agonist for a cytokine which stimulates T-cell activation In some embodiments the at least immune stimulating agent is an antagonist of a chemical, such as CXCR2, CXCR4, CCR2, C In some embodiments the immune stimulating agent is an antibody. In some embodiments the immune stimulating agent can be a vaccine. For example, a mesothelin targeting vaccine or an attenu

In some embodiments the compositions may include any of the above antagonists and agonists as well as binding agents, combined with one or more anti-CSF1R antibody described herein. Compositions can include each therapeutic agent separately in a container or compartment, or two or more therapeutic agents may be mixed together.
In certain embodiments, compositions include an anti-CSF1R antigen and a CD40 antagonist, optionally with at least one immune stimulating agent, as listed above. In some embodiments the CD40-agonist is an antiCD40 antibody. In some embodiments the anti-CD40 antibodies are the CDRs from an antibody selected among CP-870.893, dacetuzumab, SEA-CD40, ADC-1013, RO7009789, and Chi Lob 7.4 In some embodiments the anti-CD40 antibodies comprises the heavy and light chain variable region of an antibody chosen from CP-870.893, dacetuzumab, SEA-CD40, ADC-1013, RO7009789, and Chi Lob 7.4 In some embodiments the anti-CD40 antibodies are selected from CP-870.893, dacetuzumab, SEA-CD40, ADC-1013, RO7009789, and Chi Lob 7.4. In some embodiments the CD40 agonist can be recombinant CD40L. In some embodiments the compositions include any of the immune stimulating agents described above, combined with any of the anti CSF1R antibodies described in this document, as well as a CD40 antagonist, such a CD40 antibody or recombinant CD40L such as any of the anti CD40 antibodies described above. Compositions can include each therapeutic agent separately in a container or compartment, or two or more therapeutic agents may be mixed together.
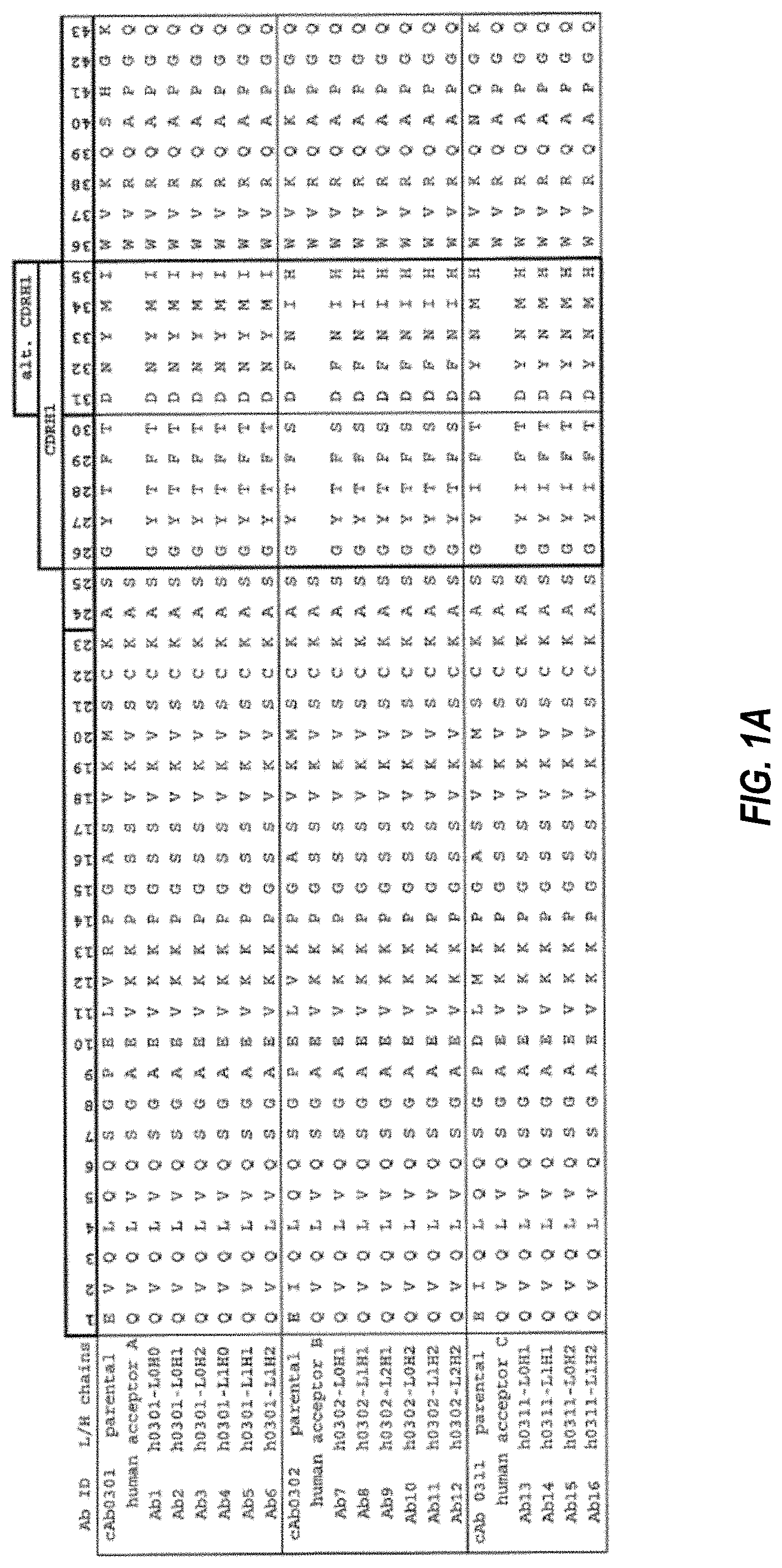
The anti-CSF1R antibodies heavy chain or light chain may be arranged as follows in any compositions or methods described.
In any of the compositions and methods described in this document, the anti CSF1R heavy chain can contain a sequence which is at least 90% identical to one selected from SEQ NOs: 9, 11-13, and 39-45. In any of these methods, the anti CSF1R light chain can contain a specific sequence that is 90%, 95%, 97%, 99% or 100% identical to the sequences selected from SEQ NOs 10, 12, 14 and 46 to52. The anti-CSF1R heavy chain can be a combination of a minimum 90%, 95%, 97% or 100% sequence from SEQ NOs 9, 11, 13 and 39 to 44. The anti-CSF1R light chain is a 90%, 95% or 97% sequence from SEQ NOs 10, 12, 14 and 46 to52.
In any of the compositions and methods described herein the anti-CSF1R antibodies HC CDR1, and HC CDR2 may contain a sequence selected from (a) the SEQID NOs 15, 16, and 17, (b) the SEQID NOs 21, 22, and 23, and (c), the SEQID NOs 27, 28, and 29, In any of compositions or methods herein described, the anti CSF1R antibodies LC CDR1, LC CDR2, LC CDR3 can comprise a sequence selected from (a) sEQ ID no. 18, 19, and 20, (b) sEQ ID no. 24, 25, and 26, and (c), sEQ ID nos. 30, 31, and 32.
The anti-CSF1R heavy chain can be composed of HC CDR1, HC CDR2, or HC CDR3. These sequences are selected from (a) the SEQ NOs 15, 16, and 17, (b), SEQ NOs 21, 22, and 23, and (c), SEQ NOs 27, 28, and 29, and the light chains may consist of LC CDR1, LC CDR2, or LC CDR3, which comprise a sequence set selected from (a) the SEQ NOs 18, 19, and 20,
The
The anti-CSF1R antibodies can be found in any of the following compositions and methods: (a) A heavy chain consisting of a CDR1 with the SEQ NO 15 sequence, an HC CDR2 with the SEQ NO 16 sequence, and an HCD3 with the SEQ NO 23 sequence; (b), a heavier chain consisting of a CDR1 with the SEQ NO 21 sequence, an HC CDR2 and SEQ NO 22 sequences and an
In some embodiments, an antibody comprises a heavier chain and a lighter chain. The antibody can consist of: (a), a heavier chain consisting of a SEQ NO:53 and a lighter chain consisting of a SEQ NO:60; (b), a heavier chain consisting of a SEQ NO:53 and a lighter chain consisting of a SEQ NO:61; (c) or a heavier chain with a SEQ NO:58 and a lightweight chain with a SEQID NO: In certain embodiments, the antibody consists of a heavier chain and lighter chain. The antibody consists of: (a), a heavier chain consisting the sequence SEQ NO:53 and a lighter chain consisting the sequence SEQ NO:60; (b), a heavier chain consisting the sequence SEQ NO:53 and a lighter chain consisting the sequence SEQ NO:61; (c) an heavy chain comprising the sequence SEQ NO:58 and a lightweight chain consisting the sequence SEQ NO
In any of the compositions and methods described in this document, the anti CSF1R antibodies may be humanized antibodies. The anti-CSF1R antibodies can be chosen from the following: a Fab or Fv, a scFv or a (Fab?)2. The anti-CSF1R may be a hybrid antibody in any of the compositions and methods described herein. The anti-CSF1R antibodies can be chosen from IgAs, IgGs, or IgDs in any compositions or methods described. The anti-CSF1R antibodies can be IgG in any of the compositions and methods described here. The antibody can be IgG1 and IgG2 in any of the described methods.

The anti-CSF1R antibodies may bind either to human CSF1R or to cynomolgus CSF1R in any of the compositions and methods described. The anti-CSF1R antibodies may prevent ligands from binding to CSF1R in any of the compositions and methods described. The anti-CSF1R antibodies can block CSF1 or IL-34 binding to CSF1R in any composition or method described here. The anti-CSF1R antibodies can block both CSF1 as well as IL-34 binding to CSF1R in any of the compositions and methods described here. The anti-CSF1R antibodies can inhibit CSF1R phosphorylation in any composition or method described here. The anti-CSF1R antibodies may inhibit CSF1 and/or IL34-induced CSF1R phosphorylation in any of the compositions described herein. The anti-CSF1R may bind human CSF1R in any of the compositions and methods described herein with a KD of less than 1nM. The anti-CSF1R antibodies may inhibit monocyte proliferative and/or survival responses when CSF1 or IL-34 is present in any of the compositions described herein.

Click here to view the patent on Google Patents.