Invented by Robert Yongxin Zhao, Hangzhou Dac Biotech Co Ltd
In the field of biotechnology and pharmaceuticals, the development of targeted therapeutics has gained significant attention in recent years. One of the key aspects of targeted therapeutics is the ability to selectively deliver drugs or imaging agents to specific cells or tissues, while minimizing off-target effects. Acetylenedicarboxyl linkers have emerged as a valuable tool in achieving this goal.
Acetylenedicarboxyl linkers are a class of chemical compounds that possess a unique property of reacting with azide groups through a copper-catalyzed click chemistry reaction. This reaction is highly efficient, specific, and bioorthogonal, meaning it can occur in the presence of biological molecules without interfering with their functions. This property makes acetylenedicarboxyl linkers ideal for conjugating a cell-binding molecule, such as an antibody or a peptide, with a drug or imaging agent.
The market for acetylenedicarboxyl linkers and their applications in specific conjugation has witnessed significant growth in recent years. The increasing demand for targeted therapeutics, coupled with advancements in click chemistry techniques, has fueled the market’s expansion. The ability of acetylenedicarboxyl linkers to selectively deliver drugs or imaging agents to specific cells or tissues has opened up new avenues for the treatment and diagnosis of various diseases, including cancer, autoimmune disorders, and infectious diseases.
One of the key drivers of the market is the rising prevalence of cancer worldwide. Cancer is a complex disease characterized by uncontrolled cell growth and the ability to metastasize to different organs. Targeted therapeutics that can specifically bind to cancer cells and deliver potent drugs or imaging agents have the potential to revolutionize cancer treatment. Acetylenedicarboxyl linkers play a crucial role in the development of such therapeutics by enabling the conjugation of cell-binding molecules, such as antibodies or peptides, with chemotherapeutic drugs or imaging agents.
In addition to cancer, acetylenedicarboxyl linkers have found applications in other disease areas as well. For example, in the field of autoimmune disorders, where the immune system mistakenly attacks healthy cells, targeted therapeutics can help modulate the immune response and reduce inflammation. Acetylenedicarboxyl linkers can be used to conjugate cell-binding molecules with immunomodulatory drugs, allowing for precise delivery to the affected cells or tissues.
The market for acetylenedicarboxyl linkers is also driven by the increasing demand for personalized medicine. Personalized medicine aims to tailor medical treatments to individual patients based on their genetic makeup, lifestyle, and other factors. Acetylenedicarboxyl linkers enable the development of targeted therapeutics that can be customized to specific patient populations, thereby improving treatment outcomes and reducing side effects.
Furthermore, the market for acetylenedicarboxyl linkers is supported by ongoing research and development activities. Scientists and researchers are continuously exploring new applications and improving the efficiency of click chemistry reactions. This has led to the development of novel acetylenedicarboxyl linkers with enhanced properties, such as increased stability, improved water solubility, and reduced toxicity. These advancements are expected to further drive the market’s growth in the coming years.
In conclusion, the market for acetylenedicarboxyl linkers and their uses in specific conjugation of a cell-binding molecule is witnessing significant growth due to the increasing demand for targeted therapeutics, rising prevalence of cancer, and advancements in click chemistry techniques. The ability of acetylenedicarboxyl linkers to selectively deliver drugs or imaging agents to specific cells or tissues holds immense potential for the treatment and diagnosis of various diseases. Ongoing research and development activities are expected to further propel the market’s expansion, making acetylenedicarboxyl linkers a valuable tool in the field of biotechnology and pharmaceuticals.

The Hangzhou Dac Biotech Co Ltd invention works as follows
The present invention provides cell binding agent-drug combinations containing bridge linkers as well as methods for using these linkers and conjugates.

Background for Acetylenedicarboxyl Linkers and Their Uses in Specific Conjugation of a Cell-Binding Molecule
Proteins, specifically antibodies have been extensively used in therapeutic applications, in vitro assays as research reagents and in vivo as diagnostic tools or as therapeutic drugs (Gad, S. C. Drug discovery handbook, published by Wiley-Interscience, 2005). For many applications the protein needs to be modified with an interesting group, such as a cytotoxic drug, a radio label element or a chromphore molecule for use in therapy or a detection agent when used in diagnostics (Teicher, B. A. et al. Clin. Cancer Res. 2011, 17, 6389-97; Elsadek, B. et al., J. Control Release, 2012, 157, 4-28). One of these applications, called antibody-drug conjugates (ADCs), which is the exquisite targeting ability of antibodies in combination with the cytotoxic action of anticancer agents, enables to target and deliver drugs to cancer cells leaving normal cells largely unaffected, has been intensely exploitation in the last two decades. In particular, since US FDA approvals of Adcetris (brentuximab vedotin) in 2011 and Kadcyla (ado-trastuzumab emtansine) in 2013, the applications of antibody-drug conjugate (ADC) as a promise targeted treatment of cancers have been exploded and almost every major pharmaceutical and biotech company has adopted this approach (Chari, R. et al, Angew. Chem., Int. Ed. 2014, 53, 3796-3827; Sievers, E. L. et al. Annu Rev Med. 2013, 64, 15-29; Mehrling, T. Future Oncol, 2015, 11, 549). Currently there are more than 50 ADC drugs in the clinic trials according to www.clinictrails.gov.
The first-generation ADCs such as Kadcyla or Adcetris are produced by nonselective coupling of native lysine amino acids on the antibody to a cytotoxic agent. This nonselective coupling results in the random cross-linkage between cytotoxic drugs and IgG1 antibodies’ surface-exposed cysteine residues. 2005 Protein Sci. 14, 2436; Hamblett, K. J., et al. 2004 Clin. Cancer Res. 10, 7063). Some of the undesirable ADC subpopulations could result in shorter circulation half-lifes, decreased efficacy and increased off-target toxicities (Hamblett K. J., et. al., Clin. Cancer Res. 2004, 10, 7063-7070; Adem, Y. T. et al, Bioconjugate Chem. 2014, 25, 656-664; Boylan, N. J. Bioconjugate Chem., 2013, 24, 1008-1016; Strop, P., et al 2013 Chem. Biol. 20, 161-167). The batch-to-batch consistency of ADC production is challenging with this classical conjugation (Wakankar A. mAbs. 2011, 3, 161-172).
Biotechnology companies and academic institutes are therefore focusing their efforts on developing novel, reliable methods of site-specific ADC coupling. In recent years, several approaches have been developed for site-specific ADC preparation (Panowski S, 2014, MAbs 6,34). These include the incorporation of unpaired Cysteines, e.g. THIOMAB, an engineered, reactive cysteine from Genentech, is called THIOMAB (Junutula J. R. et. al. 2010 Clin. Cancer Res. 16, 4769; Junutula, J. R., et al 2008 Nat Biotechnol. 26, 925-32; U.S. Pat. Nos. Nos. Biol. 20, 161-167; U.S. Pat. No. No. Chem. 25, 569-578. US Pat Appl 20130189287 Innate Pharma, U.S. Patent. No. No. 7,893,019 to Bio-Ker S.r.l. Okeley, N. M. et. al. 2013 Bioconjugate Chem. Bioconjugate Chemistry 24, 1650; Okeley, N. M., et. al. 2013 Bioconjugate Chem. Natl. Acad. Sci. 109, 16101-16106; Zimmerman, E. S., et al., 2014, Bioconjug. Chem. 25, 351-361; Wu, P., et al, 2009 Proc. Natl. Acad. Sci. 106, 3000-3005; Rabuka, D., et al, 2012 Nat. Protoc. 7, 1052-67; U.S. Pat. No. No. 20100184135, WO2010/081110 for Sutro Biopharma; WO2006/069246, 2007/059312, U.S. Pat. Nos. Nos. 7,332,571, 7696,312, 7,638,299 and WO2007/130453 for Ambrx, U.S. Patent. Nos. Nos. 7,632,492 for Allozyne and 7,829 659 for Allozyne), The incorporation of selenocysteine in antibodies (Hofer T., et. al. 2009, Biochemistry 48: 12047-12057, U.S. Pat. No. No. Chem. 25, 1331-1341. Carrico; Isaac S. et al U.S. Pat. Nos. Nos. Chem. 25, 510-520; US Pat Appl 2014.0294867, Sanofi Genzyme). These methods produced almost homogeneous profiles of products, but required antibody-engineering and reoptimizations of cell culture conditions. The yields of genetically encoded unnatural amino acids were not high enough in most cases (Tian F. et al. 2014, Proceedings). Natl. Acad. Sci. U.S.A. 111, 1766-71), which has an impact on the price of goods for the ADC. It is also known that ADCs produced by conjugation with cysteine side chain often have limited stability when in circulation. This can lead to the premature disconnection from the cytotoxic payload prior to reaching the tumor site (Junutula J. R. et al. 2008, Nat. Biotechnol. 26, 925-32).
Pink J R and Milstein C. Nature, 1967, vol. 216, 939-941. Frangione B. Milstein C. Nature, 1967, vol. 216, 941-942. Frangione B. et. al. Biochem J 1968, 106, 15-21; Frangione B, Milstein C. J Mol Biol 1968; 33:893-906; Edelman G M, et al. Proc Natl Acad Sci USA 1969; 63:78-85; Frangione B, et al. Nature 196, 221:145-148, Spiegelberg, H. L. et al Biochemistry, 1975, 10, 2157-63). The disulfide bonds are critical to the stability and biological function of IgG molecules. Each of the four subclasses, IgG1, IgG2, IgG3, and IgG4, contains 12 disulfide intra-chain bonds. Each disulfide is associated with a specific IgG domain. In the hinge region, two heavy chains are linked by a variable amount of disulfide bonding: for IgG1 or IgG4, four for IgG2 or 11 for IgG3. IgG1’s light chain is linked to the heavy chains by a disulfide between the last cysteine of the light-chain and the fifth cysteine of the heavy-chain. For IgG2, IgG3 or IgG4, however, the light chains are linked by a disulfide between the last residue of light chain and third cysteine of heavy chain (Liu H. and K. May, 2012, mAbs, 4, 17-23). The susceptibility to disulfide bond formation in human IgG1 antibody was determined by experimental reduction, differential akylation and LC/MS analysis (Liu H et al, Anal. Inter chain disulfide bond reduction is more sensitive than intra-chain disulfide bond reduction (Chem., 2010, 82: 5219-5226). Disulfide chains between light chain and heavy chain are more susceptible than those between two heavy chains. The lower disulfide of the two inter-heavy chain disulfide bonded was more susceptible. Disulfide bond reduction was most susceptible in the CH2 region. Disulfide bond susceptibility in the VL, CL and VH domains was similar to moderate, whereas disulfide bond reduction in the CH3 region was the least. Chem., 2010, 82, 5219-5226).
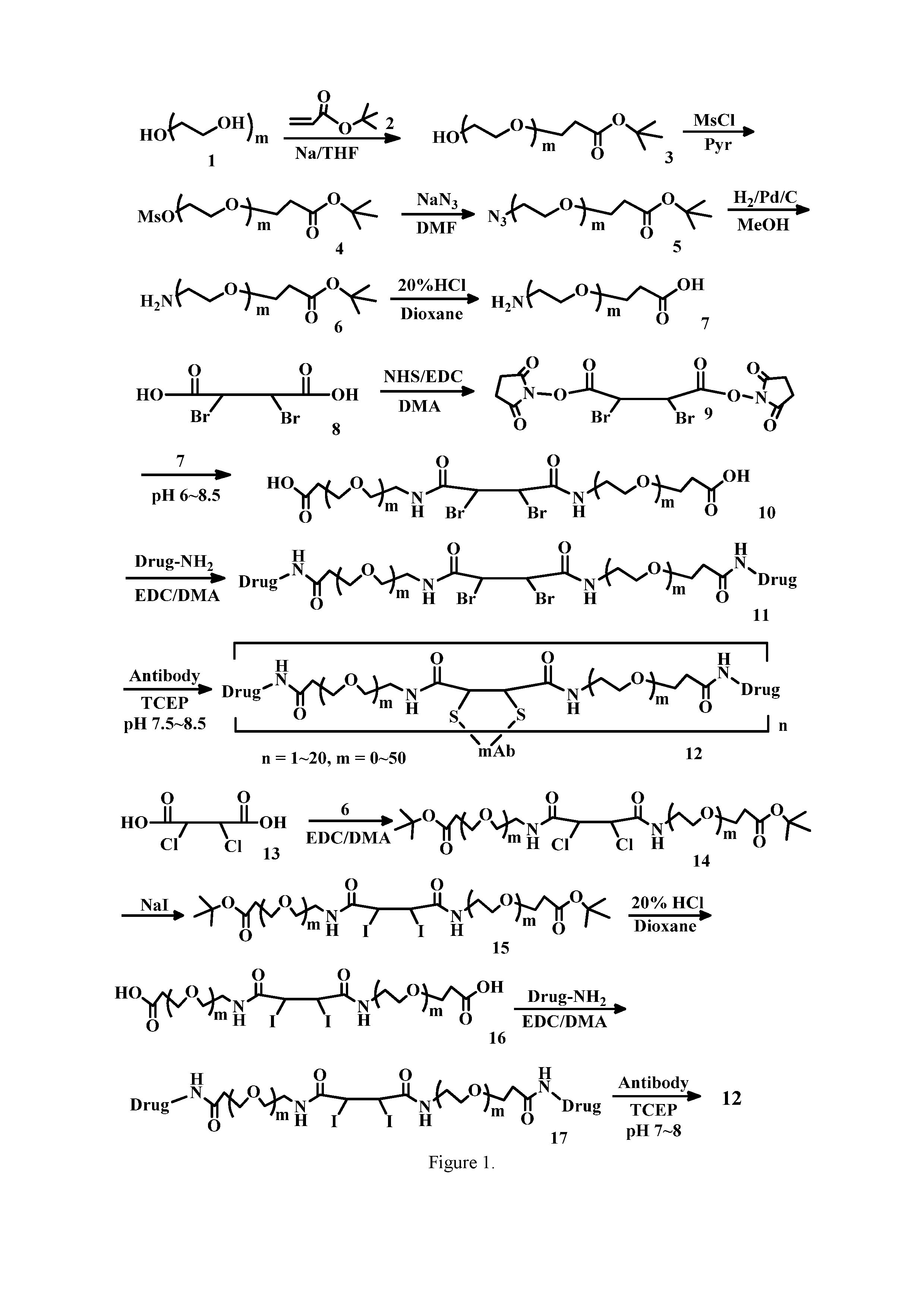
Based on the greater susceptibility of the interchain disulfide bond in human IgG1 antibody, several institutions adopted the chemically-specific conjugation strategy by rebridging the reduced interchain bonds of a Native Antibody, such as using bromo and dibromomaleimides called Next Generation Maleimides (NGMs).” (Schumacher F. F. et al. 2014, Org. Biomol. Chem. Chem. Chem. Chem. Concortis Biosystem 2015/0105539, or di-maleimide bridge (WO2014/114207). For a long time, we have used bromomaleimide and dibromomaleimide as linkers to combine drugs and antibodies (WO2014/009774 and PCT/IB2012/053554). These bridge linkers, however, were designed to only conjugate one cytotoxic agent to a pair disulfide bond, and so they produced ADCs with DARs less than two (drugs per antibodies) most of the time.
As ADCs are limited in the amount or number of cytotoxic compounds that reach the tumors, the DAR is a key factor to improve the therapeutic index of ADCs (Epenetos). A. et al, Cancer Res., 1986, 46, 3183-3191; Chari, R. V. Acc. Chem. Res., 2008, 41, 98-107, Zhao, R. Y. et al, 2011, J. Med. Chem. Chem. The molecule is too large to reach other disulfide sites such as the reduced intra-chain disulfide links beneath antibodies. These acetylenedicarboxyl links can therefore be used to selectively bridge the pairs of free disulfide bonds on the interchain of antibodies, which are produced by overloaded DTT or TCEP, and produce an ADC with DAR (drugs/antibody) above four. The over-reduced pairs of thiols that cannot be reached by bridge linkers and, in particular, by stretch-out acetylenedicarboxyl links containing two cytotoxic drugs, can also be recoupled by an oxide. Dehydroascorbic Acid (DHAA) and Cu(II) can be used to regenerate disulfide bond at the end conjugation. These bridge linkers can be used to homogenize the production of ADCs.
The present invention provides linkers containing an acetylenedicarboxylic group to link two drugs to a cell-binding agent (e.g., an antibody). The preferred formula for the cell-binding molecules-linker drug conjugates is:
wherein Cb (cell-binding agent) is used, L (acetylenedicarboxyllinker) is a linker and n (an integer between 1 and 20), and two S elements (sulfurs from Cb) bridgely link to the linker which covalently links two or more drugs per bridge linker (L). There are several advantages to using the linker for the cell-molecule-drug combination: Maintaining the stability by cross-linking covalently (rebridging), the pairs of disulfur atoms reduced in the cell-binding agents and antibodies. The conjugation of cytotoxic drugs/agents to specific sites on a cell-binding molecules, e.g. The inter-chain disulfide bonds of IgG antibody, resulting in the homogeneous manufacture of ADC.
In one aspect of this invention, the linker can be represented by Formula I.
The acetylenedicarboxyl groups on the linkers are capable of reacting to a pair sulfur atoms in the cell-binding agents. The sulfur atoms consist of preferred pairs of thiols that are reduced by a reducing agents, such as DTT or TCEP.

Z1 and Z2 is the same or different function group which allows to react with a drug to form a disulfide (secondary or tertiary), ether, ester,thioether,thioester,peptide,hydrazone, carbamate,carbonate,amine (secondary or tertiary), imine,cycloheteroalkyane, heteroaromatic,alkoxime,
R1 is the same, R2 is different and R3 and R4 are absent. The linear alkyl has from 1-6 atoms and branched alkyls have from 3 to 6 atoms.
Additionally R1 is a chain of C, N, S, O, Si and P atoms, with a preferred 0-500 atoms. This covalently links to X1 and X2 as well as Z1 or Z2. The atoms that make up R1 and R2 can be combined in any chemically relevant way, including forming alkylenes, alkenylenes, and alkynylenes; ethers; polyoxyalkylenes; esters; amines and imines in polyamines and hydrazines and hydrazones. They may also form amides and ureas as well as semicarbazides and carbazides.
X1 and X2 can be independently selected from NH or N(R3); O, S, or CH2 while R3 may be H, linear or branched alkyl with from 1-6 carbon atoms or a cyclic or branched alkyl with 3-6 carbon atoms or linear, branched, or cyclic Alkenyl.
In another aspect of this invention, the cell-binding agents, Cb and Drug1 and Drug2, and the bridge linker have reacted on the ends:
wherein:
Cb is a cell binding agent, preferable an antibody.
The linker-drug component is conjugated with pairs of sulfur atoms in the cell-binding agent. The preferred thiol pairs are reduced by DTT or TCEP from the interchain bonds of the cell binding agent.
Drug1″ and Drug2 are the same or different cytotoxic drugs, linked via a bridge linker to the cell-binding agents by a disulfide or thioether or thioester bond, or by a peptide, carbamate or carbonate, or an ester or ether or ester, or a cycloheteroalkyane or heteroaromatic or alkoxime, or amide.
Click here to view the patent on Google Patents.