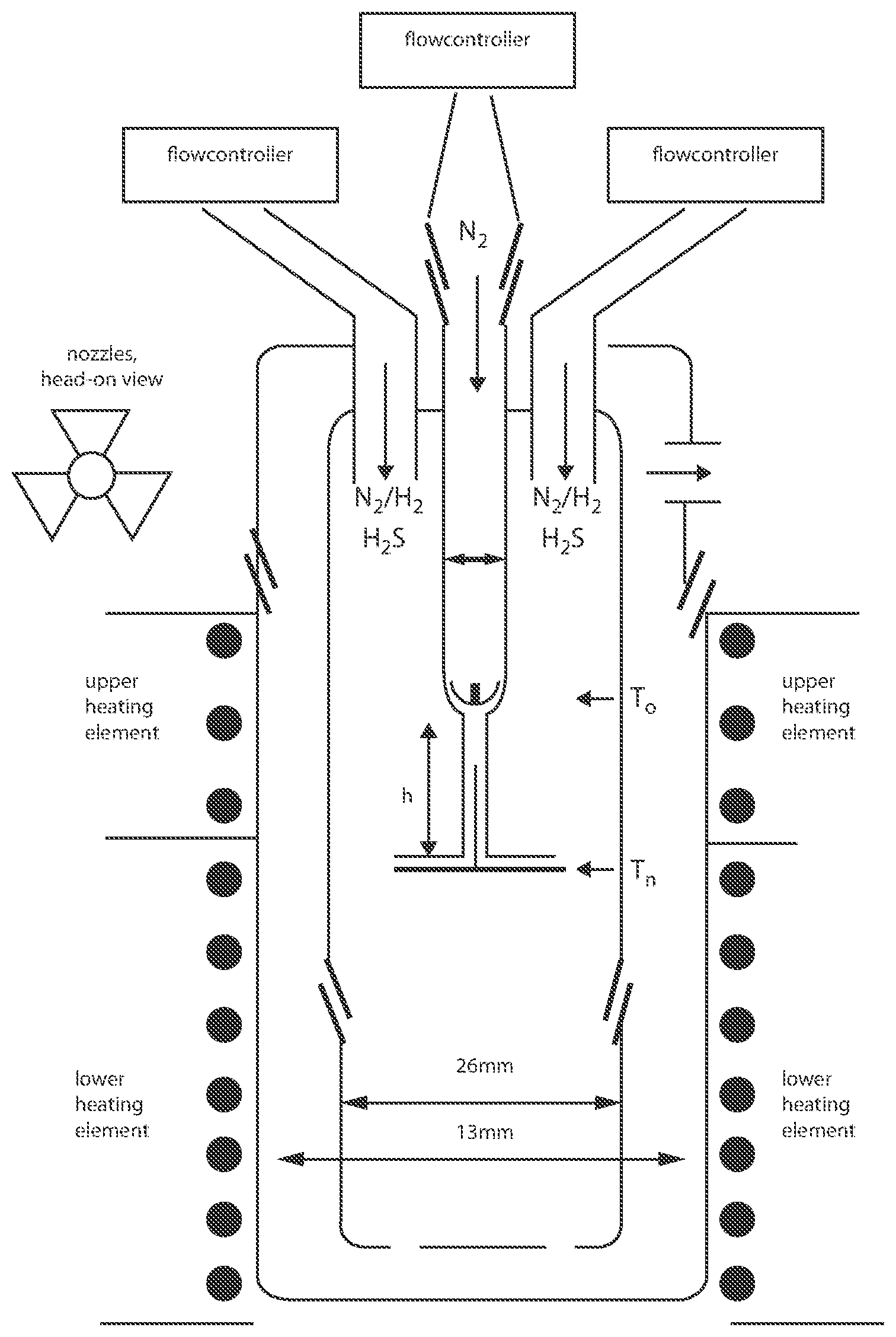
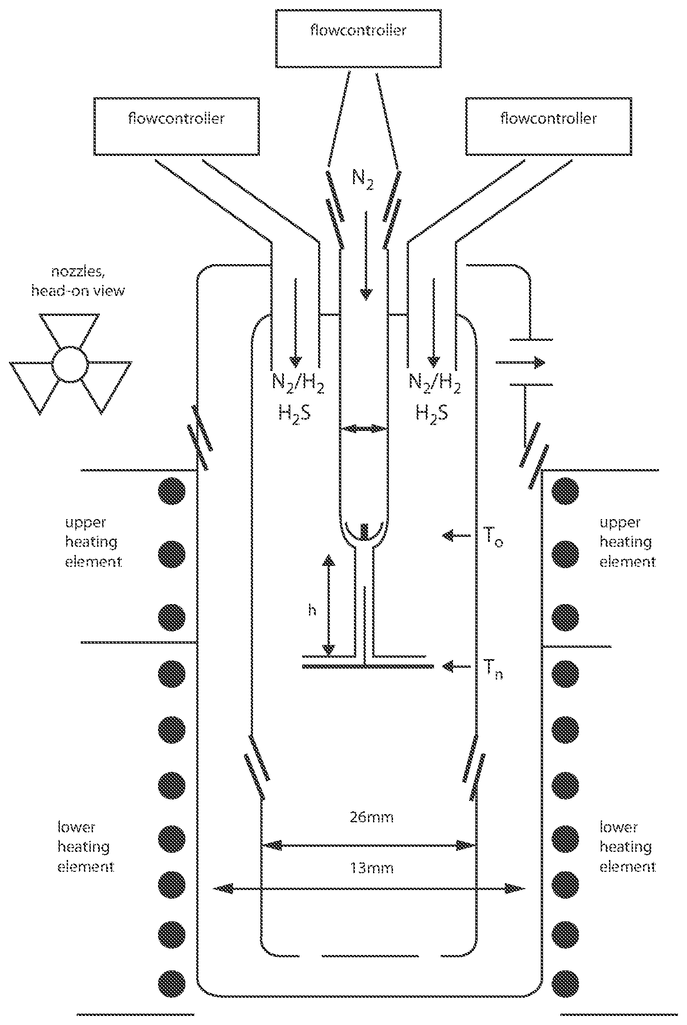
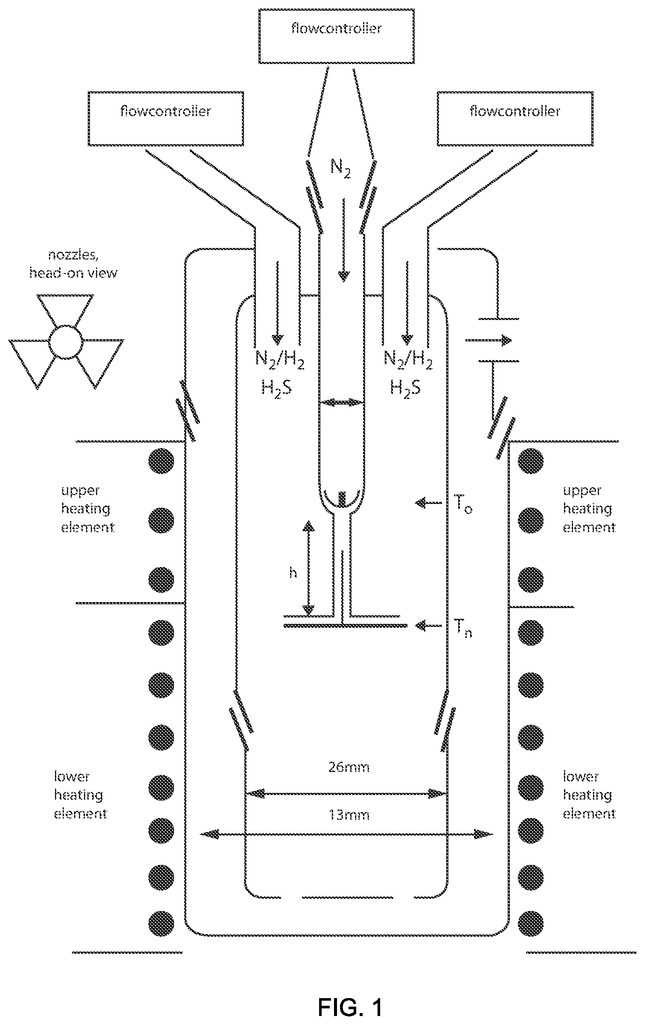
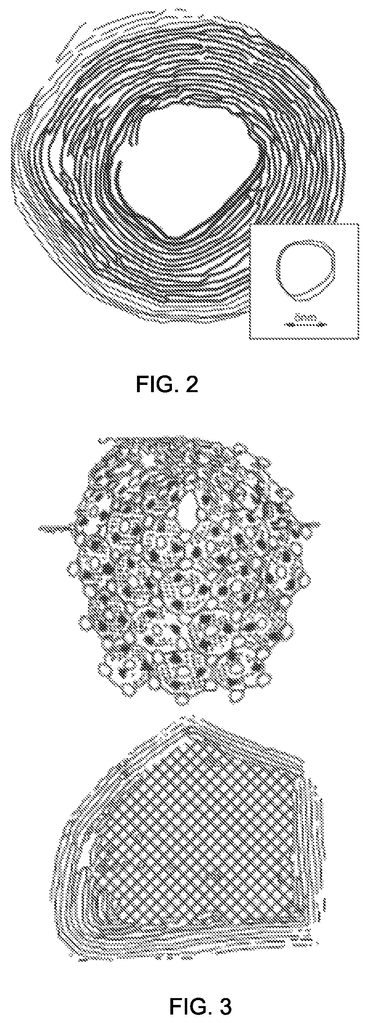
Invented by George Diloyan, Girija S. Chaubey, Debapriya Das, Nanotech Industrial Solutions Inc
The market for water-based nanoparticles dispersion is expected to grow significantly in the coming years, driven by the increasing demand for eco-friendly and sustainable products. The use of water-based dispersions reduces the use of harmful solvents and chemicals, making them a more environmentally friendly option.
One of the major applications of water-based nanoparticles dispersion is in the coatings industry. These dispersions are used to create high-performance coatings that offer excellent durability, scratch resistance, and UV protection. They are also used in the production of anti-corrosion coatings, which are widely used in the automotive and construction industries.
The electronics industry is another major consumer of water-based nanoparticles dispersion. These dispersions are used in the production of conductive inks, which are used in the printing of electronic circuits. They are also used in the production of transparent conductive films, which are used in touch screens, solar cells, and other electronic devices.
The healthcare industry is also a significant consumer of water-based nanoparticles dispersion. These dispersions are used in the production of drug delivery systems, which are used to deliver drugs to specific areas of the body. They are also used in the production of medical implants, which require high biocompatibility and durability.
The market for water-based nanoparticles dispersion is highly competitive, with several players operating in the market. Some of the major players in the market include BASF SE, Evonik Industries AG, and Arkema SA. These companies are investing heavily in research and development to develop new and innovative products to meet the growing demand for water-based nanoparticles dispersion.
In conclusion, the market for water-based nanoparticles dispersion is expected to grow significantly in the coming years, driven by the increasing demand for eco-friendly and sustainable products. The use of water-based dispersions reduces the use of harmful solvents and chemicals, making them a more environmentally friendly option. The coatings, electronics, and healthcare industries are the major consumers of water-based nanoparticles dispersion, and several players are operating in the market, investing heavily in research and development to develop new and innovative products.
The Nanotech Industrial Solutions Inc invention works as follows
A water-based dispersion containing lubricating particles.” At least one intercalation metal chalcogenide nanoparticle in water base can provide the lubricating particles. The at least one nanoparticle can have a fullerene like geometry, a tubular structure or a substantially spherical shape. Or the intercalation particles may contain particles with each of these geometries. The surface of the intercalation nanoparticles is treated with a water-soluble dispersant containing a polar functional groups.
Background for Water-based nanoparticles dispersion
Technical Field
The present disclosure is related to water-based nanoparticles, and in certain embodiments, relates to lubricants that are water-based or water-soluble (fully or partially) used in applications such as metalworking, hydraulic oil, etc.
Description of Related Art
Metalworking fluid is a term used to describe a variety of oils and liquids which are used to cool or lubricate metal pieces during machining, grinding, milling, etc. MWFs help reduce heat and friction, which can cause burning and smoke. MWFs help improve the quality and finish of the piece of work by removing fines, chips and swarfs from the cutting tool and workpiece surface.
In one embodiment, there is provided a water-based nanoparticle solution that contains a base of water and at least one metal chalcogenide intercalation particle in dispersion. The surface of the intercalation nanoparticle has been treated with a dispersant which is fully or partially water soluble and contains a polar group. The intercalation can be shaped as a platelet, spherical, near-spherical, multi-layered fullerene, tubular, or any combination of these.
The metal chalcogenide nanoparticles can be composed of a metal intercalation compound having a molecular formula of MX. M is selected from a group of metallic elements including titanium (Ti), chrome (Cr), vanadium(V), manganese(Mn), iron, cobalt, nickel, copper, zinc, zirconium, ruthenium, rhodium, palladium, palladium, cadmium, cadmium, c Metal chalcogenide compounds include molybdenum (MoS2) and tungsten (WS2). The intercalation is present at a concentration of more than 0.1 weight percent.
In some embodiments, the dispersant/surfactant that functionalizes the outer layer of the multi-layered fullerene-like nanostructure or near spherical like nanostructure is an ethoxylated phosphate ester, such as polyoxyethylene nonylphenyl ether phosphate, polyethylene glycol branched nonylphenyl ether phosphates, polyoxyethylene tridecyl phosphate ester, complex alkyl phosphate ester, or a combination thereof. In another embodiment, the dispersant/surfactant that functionalizes the outer layer of the multi-layered fullerene-like nanostructure or near spherical geometry nanoparticle is an isopropanol amine, such as diisopropanolamine, triisopropanolamine, monoisopropanolamine and combinations thereof. In another embodiment, the dispersant/surfactant that functionalizes the outer layer of the multi-layered fullerene-like nanostructure or near spherical geometry nanoparticle is an alkylalkonolamine compound such as, dimethylethanolamine, N-methyldiethanolamine, monomethylethanolamine, butylethanolamine, aminomethylpropanol, bis-(hydroxyethyl) methylamine, N,N-dimethyl-2-(2-aminoethoxy)-ethanol and combinations thereof. In another embodiment, the dispersant/surfactant that functionalizes the outer layer of the multilayered fullerene-like nanostructure or near spherical geometry nanoparticle is an ethanolamine, such as monoethanolamine, diethanolamine, triethanolamine and combination thereof.
The fullerene-like nanostructure can also be functionalized with dispersing agent consisting polar group of thiols and organic acid such as 11-mercaptoundecanoic acid, organic polysiloxane, sodium oleate soap, triethanolamine oleate, fatty alcohol polyethylene glycol ether, polyethylene glycol octyl phenyl ether, diol such as 5-decyne-4,7-diol, 2,4,7,9-tetramethyl. The surfactants may be cationic, anionic or nonionic. They can also be copolymers. polymers. monomers.
In some embodiments, industrial lubricants may be used as metalworking fluids, cooling fluids, drilling muds, gear oil, hydraulic oil, turbine oil, fire extinguishing fluids, semiconductor materials, or a mixture thereof.
In another aspect, the present disclosure describes a metalworking method using a fluid described above. Metal working methods may involve providing a metal surface and applying an industrial oil to it. The industrial lubricant may contain a water-base and at least one intercalation of a metal oxide in dispersion. Metal substrates can be preformed blank shapes for threading, metal sheets, metal plates, or combinations thereof. The intercalation material can be layered, tubular, spherical, near-spherical, or have any combination of these. The metal substrate can be worked after the industrial lubricant has been applied. The metal substrate can be worked by cutting, burning, turning, milling or grinding, sawing and threading.
In another aspect, the method for producing a water-based dispersion that includes at least one metal chalcogenide intercalation particle. The intercalation particle may have a shape that is platelet-shaped, spherical-shaped, near-spherical-shaped, multi-layered, fullerene like, tubular-like, or any combination of these. In some embodiments, the formation of the dispersion can include mixing a water base with a dispersant, wherein said dispersant includes a polar group and is at least partly soluble in the water. The dispersant is added to the water base, along with an intercalation metal chalcogenide nanoparticle having a fullerene or tubular shape, or near-spherical geometry.
Detailed embodiments” of the present disclosure have been described. However, the disclosed embodiments should be understood as merely indicative of the compositions and structures, or methods, of the disclosure, which may be embodied under various forms. Each of the examples provided in conjunction with the different embodiments is intended to be indicative, not restrictive. The figures may not be to scale and some features can be exaggerated in order to highlight details. The structural and functional details are not intended to limit, but rather to teach a person skilled in the art how to use the compositions and structures disclosed. Referring to “one embodiment”, “an embodiment”, “an example embodiment” etc. in the specification indicates that an embodiment may include a certain feature, structure or characteristic. However, not all embodiments will include this feature, characteristic or structure. These phrases do not always refer to the same embodiment.
In one embodiment, a composition for industrial lubricants is provided, comprising a water base, at least one metal chalcogenide intercalation particle in dispersion and a surface-treated dispersant, which is at least partly water soluble and, in some embodiments, is fully water soluble and has a polar group. The intercalation can have a shape that is platelet-shaped, spherical-shaped, near-spherical-shaped, multi-layered fullerene like geometry, tubular-like, or any combination of these.
The intercalation nanoparticle in the water-based dispersion exhibits at least some lubricating characteristics. The industrial lubricant can be used for metalworking fluids, drilling muds, gear oils (hygroscopic), hydro oil (hygroscopic), turbo oil (hygroscopic), heavy machine oil, machine oil, and light machine oil. The compositions disclosed in some embodiments may also be suitable as cooling fluids or semiconductor materials.
The industrial lubricant disclosed herein is water-based. The term “water-based” is used. The term “water-based” as used in the present document indicates that water is included as a solvent/medium within the lubricant. Water, for example, may be the primary ingredient in the industrial lubricant formulations disclosed herein. The compositions can include more than 50% weight in some embodiments. % water. In other embodiments the compositions can contain more than 75 wt. % water. In other embodiments, compositions from the present disclosure can include more than 90 wt. % water. Water may equal 90 wt. in some examples of water-based lubricant formulations disclosed herein. %, 91 wt. %, 92 wt. %, 93 wt. %, 94 wt. %, 95 wt. %, 95.25 wt. %, 95.5 wt. %, 95.75 wt. %, 96 wt. %, 96.25 wt. %, 96.5 wt. %, 96.75 wt. %, 97 wt. %, 97.25 wt. %, 97.5 wt. %, 97.75 wt. %, 98 wt. %, 98.25 wt. %, 98.5 wt. %, 98.75 wt. %, 99 wt. %, 99.25 wt. %, 99.50 wt. %. 99.75 wt. %, 99.8 wt. %, 99.85 wt. %, 99.875 wt. % and 99.9 weight % as well as all values between these two values as well as ranges including a limit upper and limit maximum value as provided by the examples above.
In some embodiments the industrial lubricant can be a colloidal lubricant, which is a homogeneous system made up of a dispersed phase, and a dispersion medium. In the colloidal lubricant of the present disclosure, one substance is dispersed in nanoscale particles (intercalation nanoparticles of a metal-chalcogenide) within another substance, called the dispersion media, which is water based liquid.
In some embodiments, an industrial lubricant reacts with a surface-reacted nanoparticle of a metal chalcogenide, and the tail may be hydrophilic. Industrial lubricants of the present invention include a dispersant which reacts with nanoscale particles, i.e. intercalation of a metallic chalcogenide. This creates a surface charge, which acts as a repellent force to adjacent nanoparticles, i.e. adjacent intercalation of a metallic chalcogenide. The dispersant can, for example, act as a surfactant in order to adjust the outer layer charge of the nanoscale particles, i.e. intercalation nanoparticles of a metallic chalcogenide. The dispersant can, for example, produce a negatively charged outer surface layer on the intercalation metal chalcogenide nanoparticle, such as tungsten disulfide (WS2) fullerene-like layered nanoparticles. The nanoscale particle, i.e. the intercalation of a metallic chalcogenide would repel each other if they all had the same charge. The repelling force keeps the nanoparticles dispersed in solution, by preventing agglomeration.
The intercalation particle may be composed from a metal-chalcogenide with a molecular formula of MX. M is selected from a group of metallic elements including titanium (Ti), chrome (Cr), vanadium(V), manganese(Mn), iron, cobalt, nickel, copper, zinc, zirconium, ruthenium, rhodium, palladium, silver, cadmium, hafnium, tantalum, tungsten The intercalation particle has a typical fullerene or tube-like shape or spherical/near-spherical shape, but can also have a platlet-like form. The intercalation particle can have a geometric shape that is either a platelet-shaped geometry or a spherical-shaped geometry. It may also be a multilayered fullerene like geometry, tubular-like, or a combination of these. Examples of metal chalcogenide nanoparticles include tungsten (WS2), and molybdenum (MoS2).
As used in this document, the term “fullerene like” is used. Denotes a geometry that is essentially spherical. In some cases, fullerene structures can be perfectly round, or a sphere. The metal chalcogenide structures described herein are spherical, as opposed to metal chalcogenide microstructures which may be oblong or oval (e.g. open ended oval), columnar, plate-like, or irregularly shaped particles. These particles typically result from physical methods of particle reduction, such milling from macro- and micron-scales down to nanometer-scales. Or the milling from a nanoscale particle size to a smaller nanoscale particle size.
The inorganic materials with the metal chalcogenide and fullerene or tubular geometry (i.e. intercalation nanoparticles) may be also formed according to at least one method disclosed in U.S. Patent Application Publication No. 2006/0120947, U.S. Pat. Nos. 7,524,481, 6,217,843, 7,641,869, U.S. Patent Application Publication No. 2010/0172823, U.S. Pat. Nos. 6,710,020, 6,841,142, 7,018,606, 8,513,364, 8,329,138, 7,959,891, 7,018,606, U.S. Patent Application Publication No. 2013/0109601, U.S. Patent Application Publication No. No. 2010/0227782 et U.S. Pat. No. The entire contents of each patent are incorporated in the present invention. Inorganic materials with metal chalcogenide and fullerene or tubular-like geometries can be formed by the described methods to have very small particle sizes. The methods described in the patents above are just some of the many methods suitable for the formation of inorganic materials with the metal chalcogenide and fullerene and/or tubeular-like geometry, and/or spherical or near-spherical geometries. “Any method can be used to form the inorganic materials with the metal chalcogenide, as long as the compound has a fullerene and/or tube-like geometry.
Click here to view the patent on Google Patents.