Invented by Richard L. Skelly, Judy Firor, AstraZeneca UK Ltd
Dihydropyridine-type calcium channel blockers are a class of drugs that work by relaxing the blood vessels, allowing blood to flow more easily and reducing the pressure on the walls of the arteries. They are commonly used to treat hypertension and other cardiovascular conditions, such as angina and arrhythmias.
The market for methods to lower blood pressure using a pharmaceutical composition of dihydropyridine-type calcium channel blockers is growing rapidly. According to a report by Grand View Research, the global market for calcium channel blockers is expected to reach $15.5 billion by 2025, driven by the increasing prevalence of hypertension and the growing demand for effective treatments.
One of the key factors driving the growth of this market is the increasing awareness of the health risks associated with high blood pressure. As more people become aware of the dangers of hypertension, they are seeking out effective treatments to help lower their blood pressure and reduce their risk of heart disease and stroke.
Another factor driving the growth of the market for dihydropyridine-type calcium channel blockers is the increasing availability of these drugs. There are now several different types of calcium channel blockers available on the market, including amlodipine, nifedipine, and felodipine, among others. These drugs are available in a variety of formulations, including tablets, capsules, and extended-release formulations, making them more convenient and accessible for patients.
In addition to their effectiveness in lowering blood pressure, dihydropyridine-type calcium channel blockers are also relatively safe and well-tolerated by most patients. They have a low incidence of side effects, which makes them a popular choice for patients who are looking for a safe and effective treatment for hypertension.
Overall, the market for methods to lower blood pressure using a pharmaceutical composition of dihydropyridine-type calcium channel blockers is expected to continue to grow in the coming years. As more people become aware of the health risks associated with hypertension and seek out effective treatments, the demand for these drugs is likely to increase. With their proven effectiveness and safety profile, dihydropyridine-type calcium channel blockers are well-positioned to meet this growing demand and help millions of people around the world manage their blood pressure and reduce their risk of serious health problems.
The AstraZeneca UK Ltd invention works as follows
A method for lowering blood pressure in someone who is in need of it by giving a dihydropyridine type calcium channel blocking pharmaceutical composition to someone who qualifies for over-the counter access to the dihydropyridine type calcium channel blocker pharmaceutical compound. The dihydropyridine-type calcium blocker pharmaceutical composition may include isradipine or nifedipine. In some embodiments, the dihydropyridine-type calcium channel blocker pharmaceutical composition includes 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate or a pharmaceutically acceptable salt thereof.
Background for Methods to lower blood pressure using a pharmaceutical composition of dihydropyridine-type Calcium channel blocker
Hypertension (e.g. high blood pressure) is the leading cause for death around the world and the second-leading preventable cause of death in the United States. Roberts, N., and others, Emitro Health.?Feeling Pressure of New Guidelines to the Treatment of Hypertension? (2017). According to the CDC, approximately 46% of Americans with high blood pressure were not under control in 2014. Merai R., et al., MMWR Morb Mortal Wkly Rep, 65:1261-1264 (2016). About 20% of the 35 million Americans who don’t have their blood pressure under control are aware they have high blood pressure but aren’t being treated. Id. According to the CDC, $49 billion annually is spent on direct and indirect medical costs related to uncontrolled hypertension. Id.
Fortunately, hypertension can also be managed with dihydropyridine-type Calcium channel blockers. These are well-recognized prescription drugs that lower blood pressure and prevent heart disease. Amlodipine was approved for hypertension treatment in the United States in 1992. It has been proven to be effective in lowering blood pressure in at least 15 double-blind placebo-controlled, randomized studies. Access to dihydropyridine-type calcium channels blockers is limited by the need for a prescription. Unfortunately, long-term trends demonstrate many people avoid prescription medications, including dihydropyridine-type calcium channel blockers.
One way to make dihydropyridine-type calcium channels blockers more accessible is to make them readily available, e.g., without the need for a prescription. Switching from prescription to OTC can have a number of health benefits, including the ability to provide more therapies and faster access to treatments. Patients are able to take an active role in their health and be able to prevent and treat minor conditions. (World Health Organization 2000,?Guidelines to the Regulatory Assessment of Medicinal Products For Use in Self-Medication. Print). Access to OTC calcium channel blockers of dihydropyridine type could be a significant health benefit for society, given the high number of people with uncontrolled high blood pressure.
However, the switch from prescription-only to OTC distribution of a drug creates a risk that patients will not be able to properly self-select for safe use and then self-medicate with the drug responsibly. These concerns include inaccurate self-diagnosis, wrong drug-qualifications, unrecognized drugs-drug interactions (DDI), unexpected adverse drug reactions and/or effects, improper dosing/or administration, masking a disease, addictions, inappropriate drug dependency, substance use, patient delay in seeking medical attention, and delayed access to necessary medical attention. Ruiz et al., Current Drug Safety, 5(4):315 (2010).
Prospective patients must self-select the drug in order to ensure safety during OTC distribution of dihydropyridine type calcium channel blockers. However, recent studies have shown that many potential patients fail to pay attention to the guidelines on OTC drug packaging to ensure responsible and safe use. PR Newswire Association.?Americans Should Pay more Attention to OTC Medicine Labels According To New Survey. October 15, 2015 (citing McNeil Consumer Healthcare Research). These studies show that 40% of potential patients view the directions only as guidelines, and that 80% of patients don’t re-read OTC medication labels they’ve used before. Worse, 58% of the men surveyed did not consider it important to read OTC labels.
There are currently two legal pathways that allow for the legal marketing of OTC drugs in the United States. The first pathway involves marketing in compliance with an OTC Drug Monograph. This sets the regulatory standards for nonprescription drugs not covered by human drug application. The FDA conducts a three-phase OTC drug review to create an OTC monograph. Phase I of the review is where an advisory panel decides whether the OTC composition proposed could be considered safe and effective for self-treatment. The second pathway involves marketing under an approved product-specific, new drug application (NDA) or an abbreviated, new drug application. A consumer research study is necessary to determine whether a label can be used to promote an over-the counter label for a drug that is subject to regulatory approval through an NDA. This is done based on the proposed labeling of the drug. Oliver, A., Regulatory Rapporteur. 10(3):4-9, 2013. This is incorporated herein by reference.
But, efforts to switch distribution of cardiovascular drugs that could have far-reaching benefits to society’s health from prescription-only to OTC models have failed repeatedly due to concerns about patient selection and medication. Statins that are used to lower cholesterol may be the most well-documented case.
The Background section contains information that is intended to enhance understanding of the invention’s general background. It should not be construed as acknowledging or suggesting that the information constitutes prior art known to someone skilled in the art.
Given the background, systems and methods are needed to qualify a human subject for delivery over-the-counter of a dihydropyridine type calcium channel blocker pharmaceutical composition to lower blood pressure. This could be used for treating or preventing heart disease.
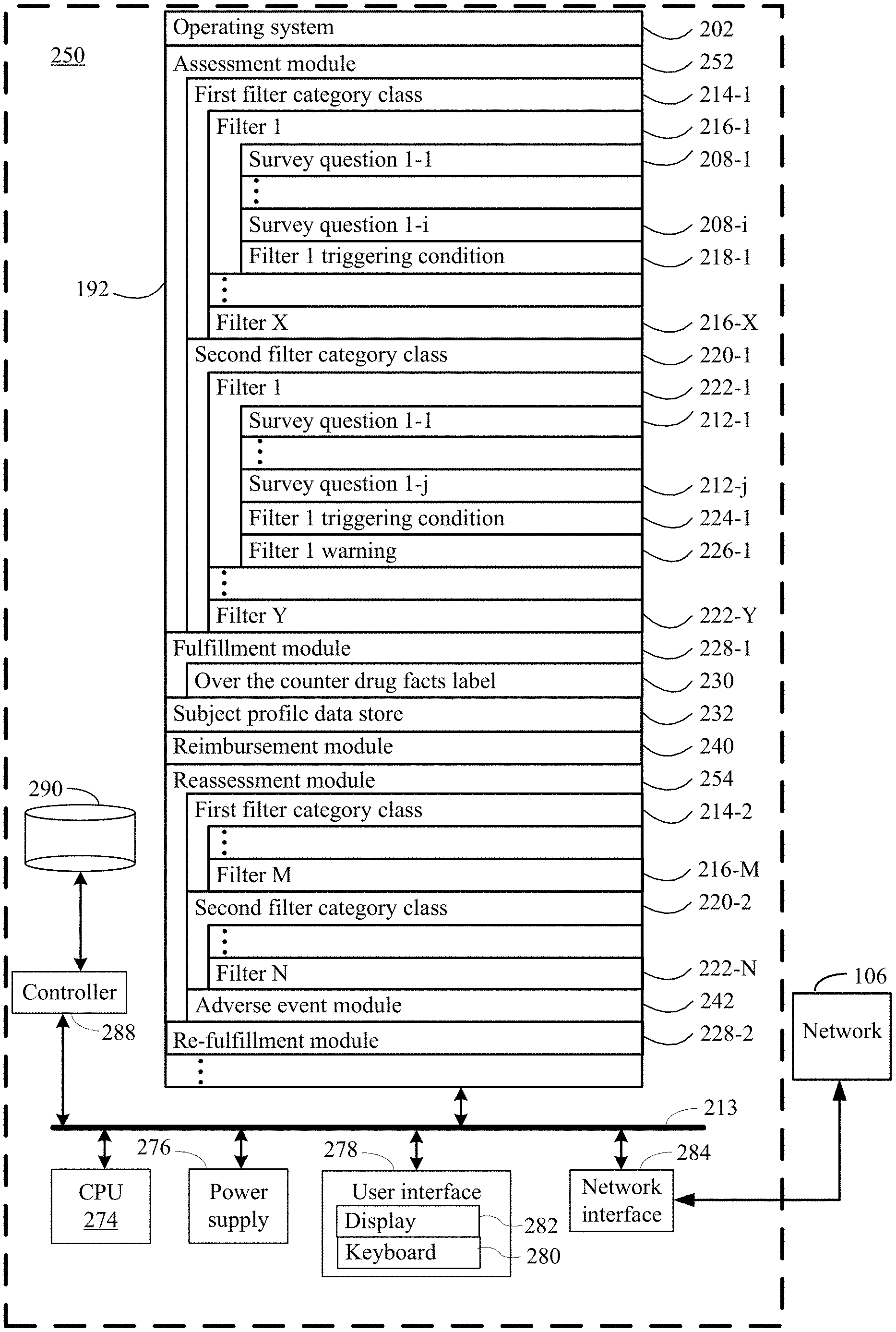
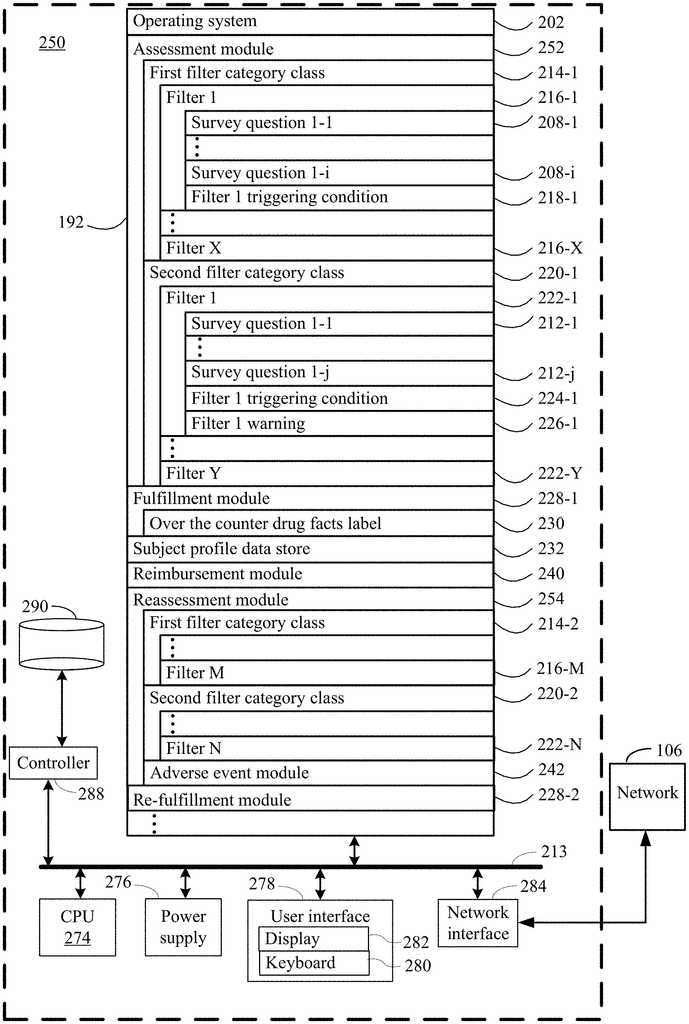
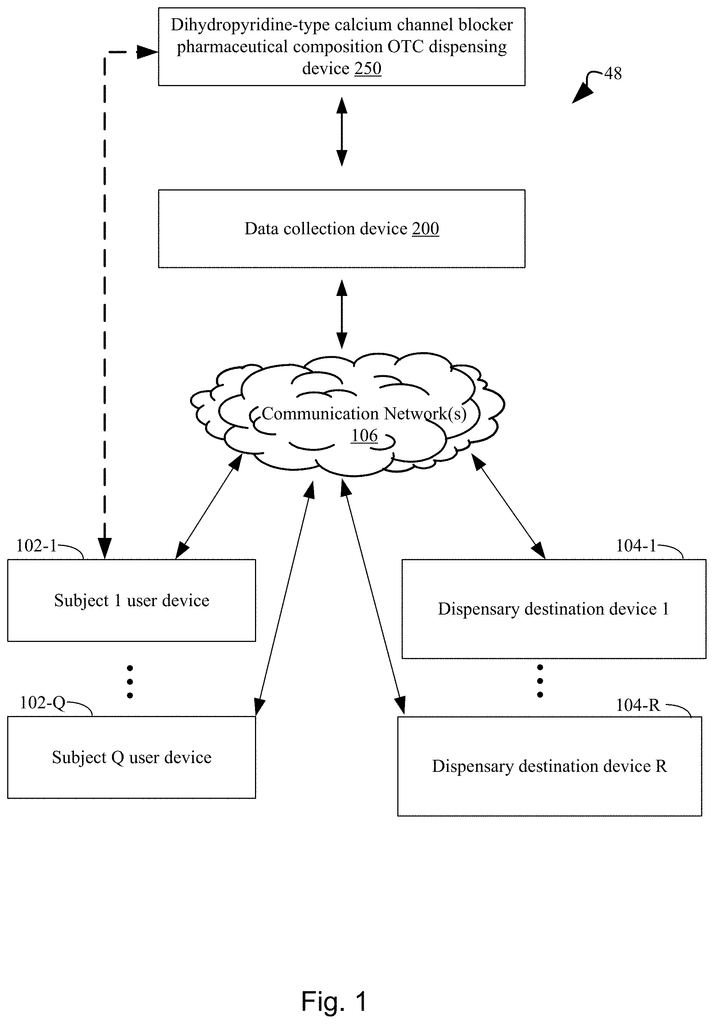
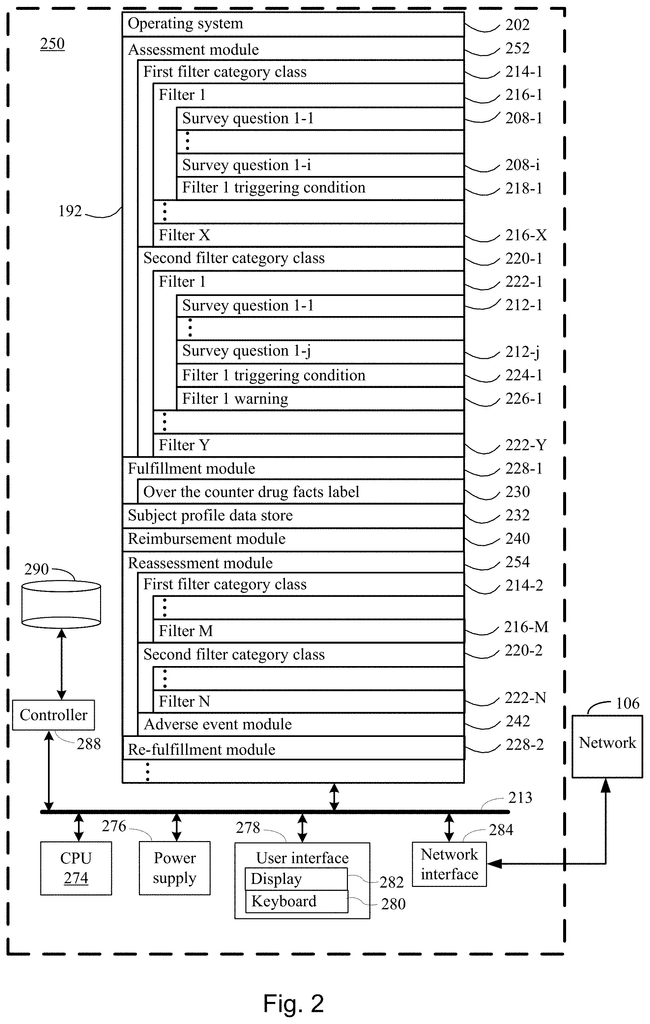
The present disclosure addresses a need in the art for systems, methods, and protocols for qualifying human subjects for over-the counter delivery of a dihydropyridine type calcium channel blocker pharmaceutical composition (e.g. amlodipine) to treat or prevent heart disease. The present disclosure provides systems and methods for the over-the-counter delivery to a subject of a calcium channel blocker pharmaceutical composition. A first plurality is used to compare survey results. The subject is not eligible for the delivery of the dihydropyridine-type Calcium Channel Blocker pharmaceutical composition if a filter from the first plurality is fired. A second plurality of filters is also used to run the survey results. The subject receives a warning when a filter from the second plurality fires. When no filter from the first plurality has been fired, the method moves to a fulfillment step. The subject has acknowledged each warning associated each filter fired in the second plurality. The fulfillment process stores the composition and communicates to the subject a drug facts label for dihydropyridine, type calcium channel blocking pharmaceutical composition. It authorizes the subject to receive the dihydropyridine, type calcium channel blocking pharmaceutical composition upon confirmation by the subject that it has been read.
According to the present disclosure, one aspect provides a method of qualifying a subject for the over-the-counter administration of a dihydropyridine type calcium channel blocker pharmaceutical composition to lower blood pressure. This method involves conducting a first survey on the subject to collect a variety survey results. The survey results may include the following: the subject’s age, gender, race, current blood pressure, current diastolic and systolic cholesterol levels, whether or not the subject is currently pregnant or breastfeeding, whether or not the subject has had any heart procedures, whether or not the subject is currently taking any dihydropyridine type calcium channel blocker pharmaceutical compositions.
The method also involves running all or part of the survey results against a plurality of filters from a first class. The subject is not eligible for the delivery of the dihydropyridine type calcium channel blocker pharmaceutical composition if a filter from the first plurality is fired. The method is terminated without the delivery of the dihydropyridine type calcium channel blocker pharmaceutical composition. Some embodiments include a first pregnancy filter and a dihydropyridine medication filters, as well as a first blood pressure filter, age filter, and a pooled cohort formula filter.
The method also involves running all or part of the survey results against a second plurality filter of a different category class. A warning is displayed to the subject when a filter from the second plurality is fired. In certain embodiments, the second plurality includes one or more of a liver disease filter or a drug interaction filter. The second plurality filters are different from the first plurality filters. They do not stop the process without delivering the dihydropyridine-type Calcium channel blocker pharmaceutical composition.
The method continues by obtaining acknowledgement from the subject for any warning issued to him by the subject by any filter within the second plurality. According to some embodiments, acknowledgment can be a written acknowledgment, a verbal acknowledgement or an electronic acknowledgment like an electronic signature.
The method continues by continuing with a fulfillment process until no filter in the first plurality has been fired, and the subject has acknowledged every warning associated with the filter in the second plurality that was fired.
In some embodiments, fulfillment includes the storage of an indication in a subject profil for an initial order of the dihydropyridine type calcium channel blocking pharmaceutical composition, communicating an under-the-counter label for the dihydropyridine type calcium channel blocking pharmaceutical composition and authorizing, after confirmation from the subject that they have read and received the label, the provision of the dihydropyridine -type calcium channels blocker pharmaceutical compound to the subject.
In certain embodiments, the dihydropyridine type calcium channel blocker pharmaceutical composition has this structure:
where,
Click here to view the patent on Google Patents.