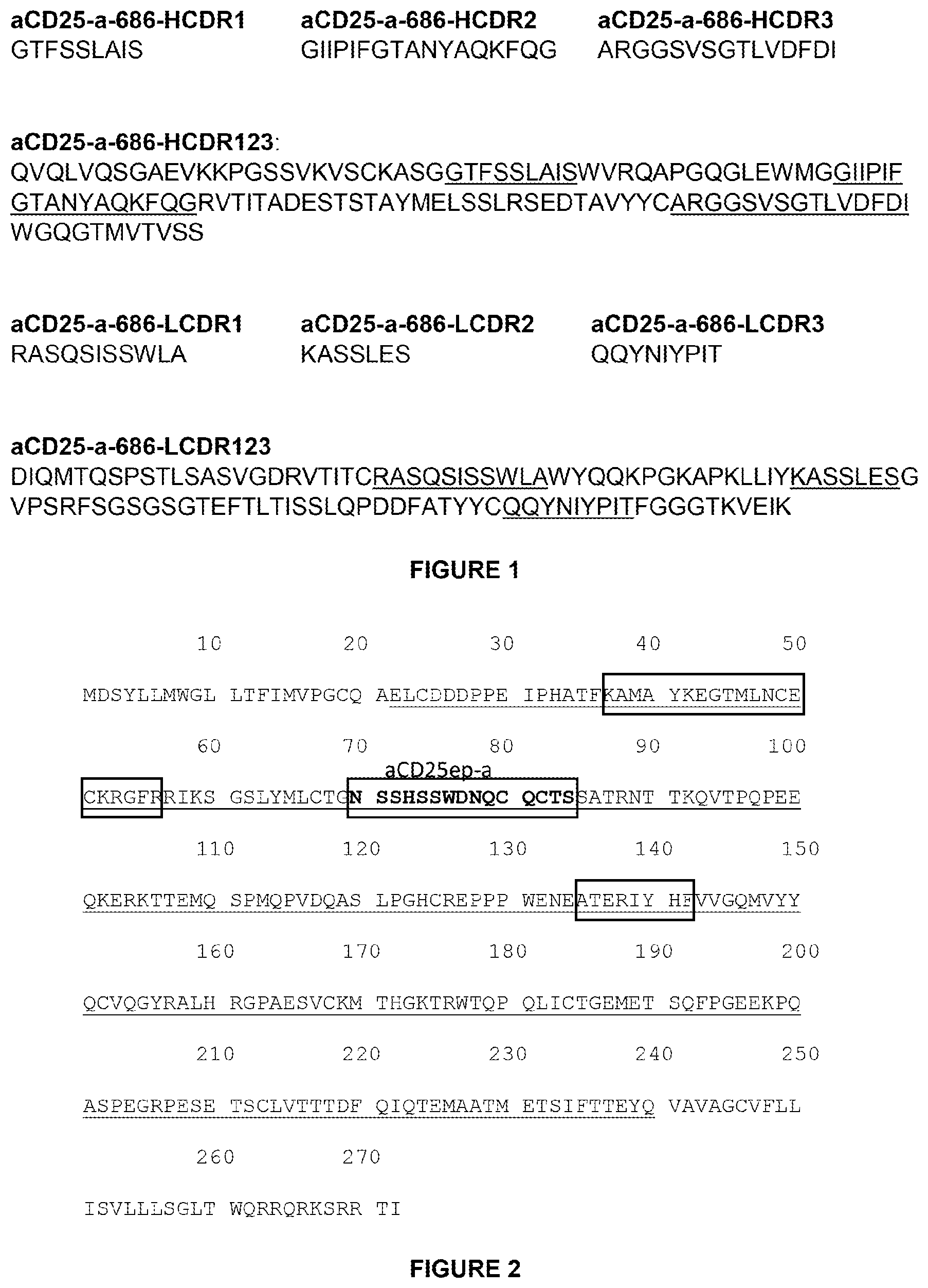
Invented by Joseph P. Balint, Frank R. Jones, Richard B. Gayle, III, Etubics Corp
The global market for adenovirus vectors is expected to grow at a compound annual growth rate (CAGR) of 9.9% from 2020 to 2027. The increasing prevalence of infectious diseases, the growing demand for gene therapy, and the rising investments in research and development activities are some of the key factors driving the growth of the market.
Several companies are actively engaged in the development of adenovirus vectors for multiple vaccinations. For instance, Vaxart Inc. is developing an oral adenovirus vector-based vaccine for COVID-19 that can provide long-lasting immunity against the virus. The vaccine is designed to express the spike protein of the virus, which can stimulate the immune system to produce neutralizing antibodies.
Another company, GenVec Inc., is developing an adenovirus vector-based vaccine for the prevention of Ebola virus disease. The vaccine is designed to express the glycoprotein of the virus, which can elicit a strong immune response against the virus.
The market for adenovirus vectors for multiple vaccinations is also witnessing significant investments from governments and private organizations. For instance, the Coalition for Epidemic Preparedness Innovations (CEPI) has invested $8.4 million in the development of an adenovirus vector-based vaccine for COVID-19. The vaccine is being developed by the University of Oxford and AstraZeneca.
In conclusion, the market for methods, compositions, and methods for creating an adenovirus virus vector for multiple vaccinations is witnessing significant growth due to the increasing prevalence of infectious diseases, the growing demand for gene therapy, and the rising investments in research and development activities. With the development of more effective and safe adenovirus vector-based vaccines, the market is expected to witness further growth in the coming years.
The Etubics Corp invention works as follows
Methods to generate immune responses using the adenovirus Vectors are provided that allow multiple vaccinations with one adenovirus virus vector, and vaccinations of individuals with preexisting immunity.
Background for Methods, compositions and methods for creating an adenovirus virus vector for multiple vaccinations
Technical Field
The invention concerns methods of generating immune responses using Adenovirus vectors that allow multiple vaccination regimens.
Description of Related Art
The greatest problem with adenovirus-based vectors is their inability to maintain long-term transgene expression. This is due to the host immune response, which eliminates the virus vector and transduces cells in immune-competent patients. Preexisting or inducible immunity to vaccines against adenovirus (Ad), limits the use of First Generation vector vaccines. J Virol 77/799-803 (2003); Casimiro, et al. J Virol 77/6305-6313 (2003)). One study found that most people have antibodies against Ad5 (the most common serotype for gene-transfer vectors), and that about two-thirds have lymphoproliferative responses to Ad (Chirmule et al. Gene Ther 6/1574-1583 (1999). Another study showed that an adenovirus vector vaccine containing an HIV-1 envelope gene was incapable in reimmunizing primed immune responses using non-adjuvanted genetic DNA. (Barouch, and al. J. Virol 77/8729-8735 (2003)). Another study reported that nonhuman primates with pre-existing immunity to Ad5 were unable, after a single Ad5 immunization, to produce transgene-specific antibodies against HIV proteins. J Virol 81/6594-6604 (2007)).
Preexisting immunity can interfere with the effectiveness of adenovirus vector vaccinations. The simplest mechanism is the presence and elimination of Ad-infected antigen-hosting cells by cell-mediated immune suppression. These responses target several Ad proteins. There are many ways to overcome preexisting anti-vector immune system. The easiest approach is to increase the dose of vector vaccine. There is evidence to suggest that increasing vaccine doses can induce desired cell-mediated immune (CMI), responses in Ad-immune animal models (Barouch et al. J. Virol 77/8729?8735 (2003). However, it can often cause unacceptable adverse reactions in humans and animals. Most investigators who use First Generation Ad5 Vector Vaccines follow a heterologous priming-boost protocol, which uses naked (non-vectored DNA) as the priming vaccine, and then an Ad5 vector vaccination. The protocol results in an immune response against Ad5 that is so strong that it cannot be used to administer another re-immunization or boost with the same (or different) adenovirus vector vaccination that uses the same viral backbone. This approach, which is the First Generation of Ad5 Vectors, also eliminates any future Ad5 vector immunizations in the Ad5 immunized vaccinee.
First Generation (E1 removed) adenovirus vector vaccinations express Ad late gene, but at a lower level and for a longer period of time than wild-type Ad viruses (Nevins, al. Cell 26/213-220 (1981); Gaynor, et al. Cell 33/683-693 (1983); Yang, et al. J Virol 70/7209-7212 (1996)). First Generation adenovirus vectors are used for immunization. Vaccine antigens are simultaneously presented to the immune system with highly immunogenic Ad capsid protein. These adenovirus vectors have the problem that the immune system is less likely to respond to vaccine epitopes. Nat Rev Immunol 2283-291 (2002). The immune responses generated by these adenovirus vectors are less likely to be directed at the desired vaccine epitopes (McMichael, et al. It is not clear how First Generation adenovirus vectors can be potent immunogens. The Ad capsid composition or the toxic effects of viral genes may cause generalized inflammation that results in an immune stimulatory effect. Ad’s E1 proteins act to prevent inflammation after infection (Schaack et al. PNAS 101/3124-3129 (2004) The removal of these gene segments, as is the case with First Generation adenovirus vectors results in an increase in inflammation (Schaack et al. PNAS 101/3124-3129 (2004); Schaack, et al. Viral Immunol 18/79-88 (2005)). Jooss et al. reported that adenovirus vectors infect immunogenic viruses more efficiently than less immunogenic ones. J Virol 72/4212-4223 (1998)). CMI responses are initiated by antigen-presenting cells (APCs), such as dendritic and dendritic cells (Kirk, Kirk, et. al. Hum Gene Ther 11/797-806 ((2000)). Hartigan-O’Connor et al. reported that preventing gene expression in dendritic cell receptors greatly reduces the intensity CMI response. Mol Ther 4/525-533 2001 )).
It is evident that there is a need for an effective vaccine vector candidate. Ad vaccine vectors must be able to allow multiple vaccinations as well as vaccinations of individuals with preexisting Ad immunity. There is also no homologous vaccine delivery channel that can be used in a primary reimmunization protocol. This and other benefits are provided by the present invention.
One aspect of the invention relates to a method for generating an immune reaction against one or multiple target antigens in an individual. This involves administering an adenovirus virus vector to an individual that contains: a) a replication defect adenovirus Vector and b) a nucleic acids encoding one or more of the target antigens. The individual then readministers the adenovirus vaccine at least once, thereby generating an immunity response against the target antigens
Another aspect is that the invention provides a method to generate an immune reaction against one or multiple target antigens in an individual. This involves administering an adenovirus virus vector to an individual. It includes: a) a replication defect adenovirus Vector, wherein it has a deletion at the E2b region; and b) a nucleic acids encoding one or several target antigens. Then, readministering the vector to the individual.
In one embodiment, the target antigen includes an HIV protein or a human papillomavirus protein, a herpes virus protein or a Streptococcus pneumonia proteins, or an immunogenic fragment, variant, of these proteins. The HIV protein can be an HIV-gag proteins in certain embodiments. A further embodiment of the target antigen includes an antigen derived either from a Western Equine Encephalitis Virus or Venezuelan Equine Encephalitis Virus. Further embodiments include a Leishmania protein or cancer protein such Her2/Neu or WT-1. In one embodiment, the human-papillomavirus protein is E6. Another embodiment has the human papillomavirus protein E7. The target antigen can be a variant with one or more lower biological activities than the wild type target antibody. A target antigen, in particular, is a variant with reduced oncogenicity than the wild-type target antigen.
In one embodiment, the target antibody is an antigen that has been derived from an influenza virus protein or a variant or fragment thereof. The H5N1 influenza virus may also be used to generate the influenza protein. Another embodiment of the influenza virus protein can be obtained from any influenza virus. This includes but is not limited to H3N2,H9N1, and H1N1, as well as H2N2, and H7N7. The influenza virus protein can be any influenza protein in certain embodiments. This includes but is not limited to haemagglutinin and neuraminidase or matrix protein M1.
A further aspect of this invention provides a method for generating an immunity response against one of more target antibodies in an individual. This involves administering to an individual a first vector of adenoviruses, which includes: a) a replica defective vector of adenoviruses, with a deletion at the E2b area, and: b) a primer vector of adenoviruses, which contains at least one target antibody, and where the target antigen of at least one of the second vector.
The adenovirus vector in one embodiment of the methods herein is not a gutted virus. Another embodiment of the methods described herein requires that the individual have preexisting immunity against adenovirus. A further embodiment of the methods is that the at least one target antibody for each of the first and second adenovirus vectors comes from the same infectious agent. Another embodiment is that the at least one target antibody of each adenovirus vector is derived from different infectious agents.
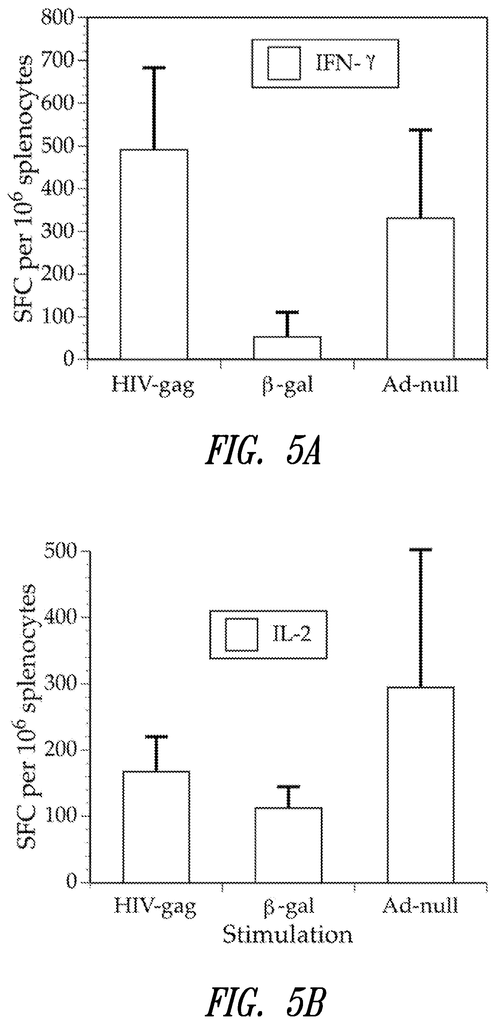
In another embodiment of the methods described in the invention, at least one target antibody of the first virus vector is an HIV protein. A further embodiment includes an HIV protein as the at minimum one target antigen in the first adenovirus virus vector and an HIV protein as the at least 1 target antigen in the second adenovirus Vector. Another embodiment includes an HIV protein as the at most one target antigen in the first Adenovirus Vector and an HIV protein as the at least 1 target antigen in the second Adenovirus Vector. A further embodiment of this invention is that the HIV protein in the first or second vectors of adenoviruses is an HIV-gag proteins. A further embodiment includes an HIV-gag protein in the first adenovirus and a second vector containing?-galactosidase in the second. In some embodiments, the ‘-galactosidase can be an E. coli??-galactosidase.
In some embodiments of the methods herein, at least one target adenovirus vector contains a cancer protein (e.g. a Her2/neu Antigen, a Human Papilloma Virus Protein or a Carcinoembryonic Protein), or a fragment thereof. Other embodiments include a bacterial, viral, antigen, antigen from a protozoan, antigen from a fungal, antigen from a mammalian, antigen from an avian, or fragment thereof. The target antigen may be a variant with one or more lower biological activities than the wild type target. A target antigen, in particular, is a variant with reduced oncogenicity than the wild-type target antigen.
A further related embodiment of the invention comprises an adenovirus virus vector. This vector includes: i) a replication defect adenovirus, wherein the vector contains a deletion at the E2b region; and ii). a nucleic acids encoding one (or more) target antigens. The target antigens include a modified cancer protein with one or several reduced activities. One embodiment of the modified cancer protein has reduced oncogenic activities. Modified cancer protein in one embodiment is a modified Her2/neu proteins with reduced kinase activity. Modified cancer protein in one embodiment is a modified E6 human papillomavirus protein that has reduced binding to p53. Modified cancer protein in one embodiment is a modified E7 human papillomavirus protein with reduced binding to Rb.
BRIEF DESCRIPTION ABOUT THE VIEWS FROM THE DRAWINGS
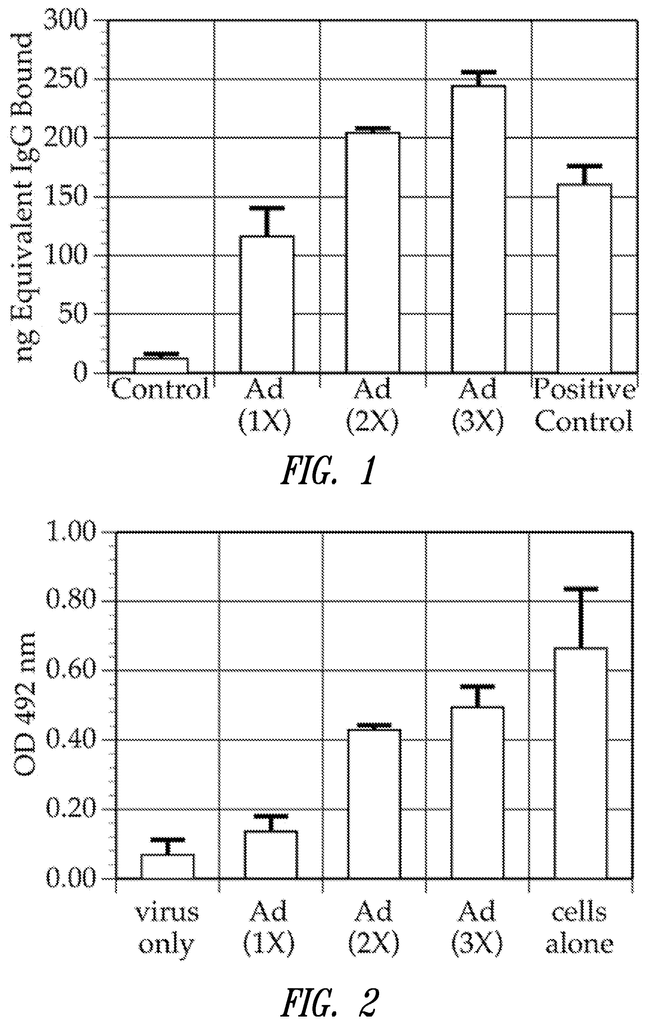
FIG. “FIG. Three times, the mice were immunized with Ad5Null virus particles at 14-day intervals. You will notice an increase in anti-Ad antibodies after each immunization.
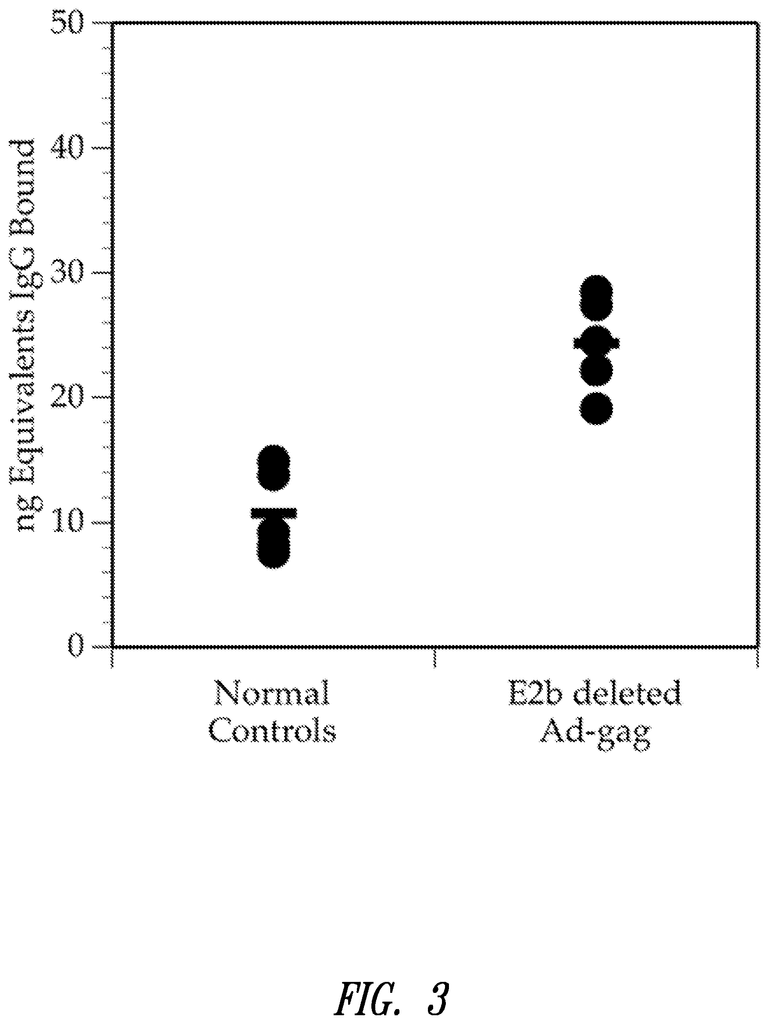
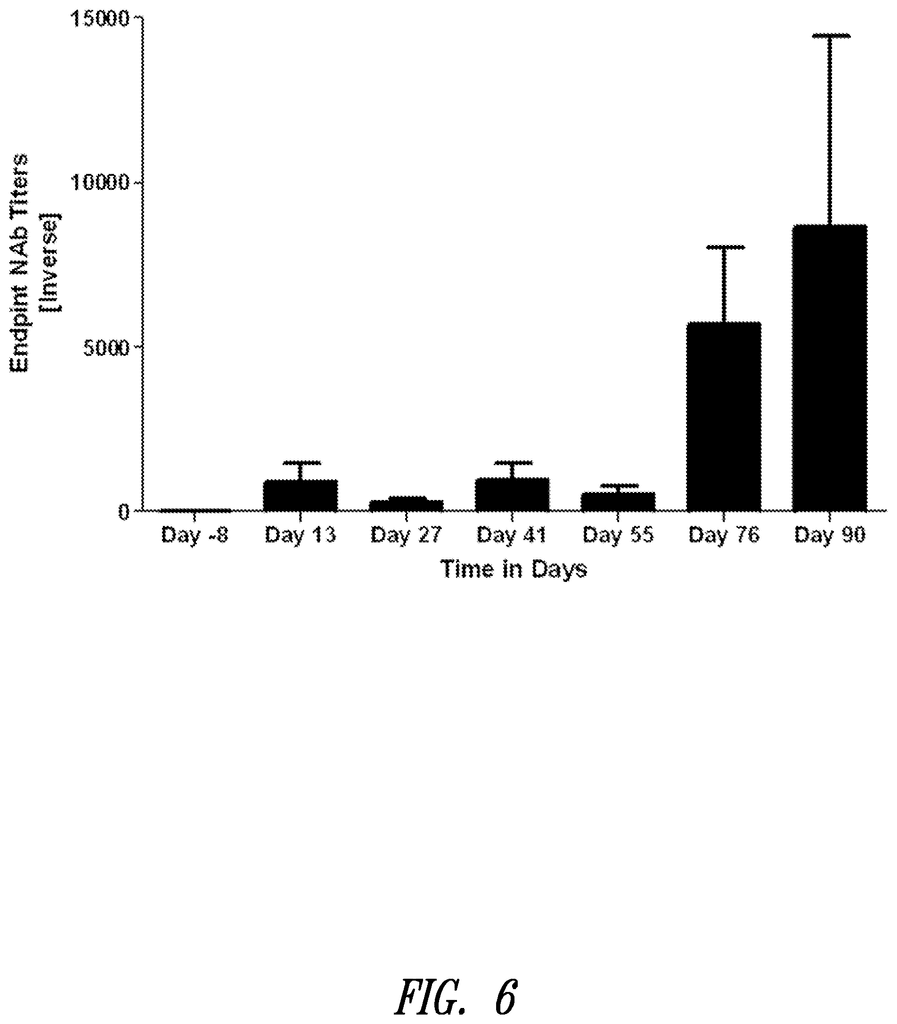
FIG. 2. A bar graph showing the neutralizing antibody levels in mice immunized by Ad5Null. Three times, at 14-day intervals, mice were immunized with Ad5Null virus particles. After each immunization, there was an increase in neutralizing antibodies. The presence of viable target cells can be determined by optical density.
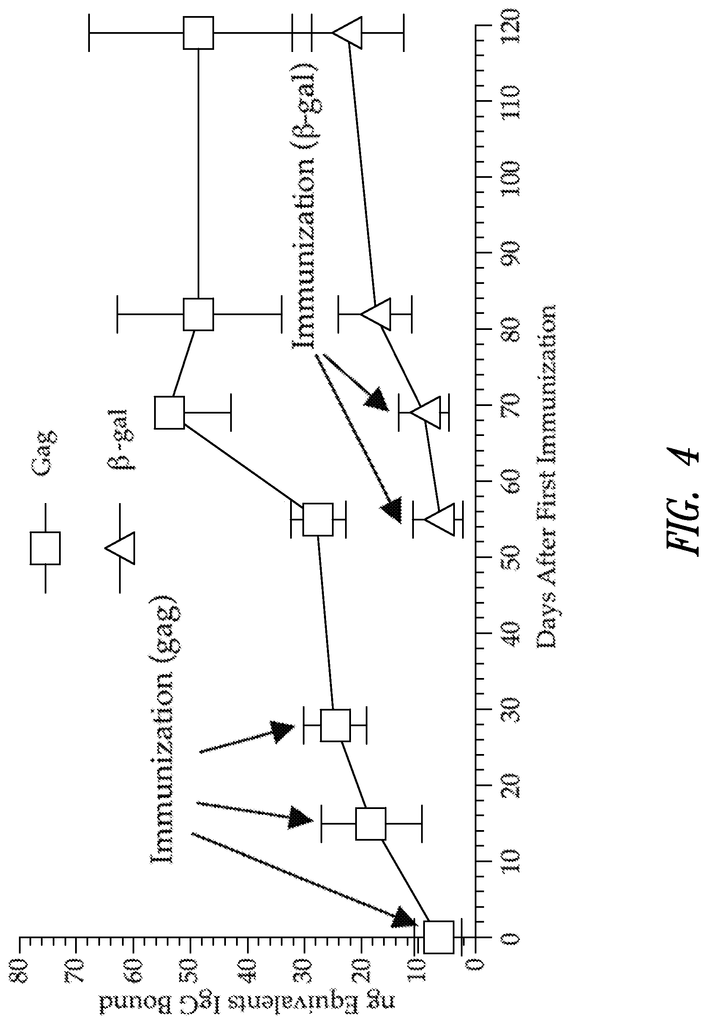
FIG. “FIG.
Mice were infected three times with E2b-destroyed adenovirus vector containing HIV-gag gene. The experimental mice had significantly higher levels (P0.05) of Gag IgG antibody than normal mice. The mean value is represented by horizontal bars.
Click here to view the patent on Google Patents.