Invented by Stefan Trentmann, Gabriele Matschiner, Arne Skerra, Andreas Hohlbaum, Martin Huelsmeyer, Hendrik Gille, Hans-Juergen Christian, Kristian Jensen, Rachida Siham Bel Aiba, Pieris Pharmaceuticals GmbH
Hepcidin is a hormone that regulates iron metabolism in the body. It is produced by the liver and acts by binding to ferroportin, a protein that transports iron out of cells. When hepcidin binds to ferroportin, it causes the protein to be internalized and degraded, leading to a decrease in iron absorption and release from cells. This mechanism is important for preventing iron overload and maintaining iron homeostasis in the body.
Hngal has been shown to bind to hepcidin and regulate its activity. Specifically, hngal can inhibit hepcidin’s ability to bind to ferroportin, leading to an increase in iron absorption and release from cells. This mechanism has potential therapeutic applications in the treatment of iron-related disorders, such as anemia and hemochromatosis.
The market for hngal muteins that bind hepcidin and nucleic acid encoding such is driven by the increasing prevalence of iron-related disorders and the need for more effective treatments. Anemia, for example, affects an estimated 1.62 billion people worldwide and is a major cause of morbidity and mortality. Hemochromatosis, on the other hand, is a genetic disorder that leads to iron overload and can cause organ damage and other complications.
There are several companies and research institutions that are currently developing hngal muteins and nucleic acid encoding such for therapeutic use. These include Novartis, which has developed a hngal-based drug called LCN2 that is currently in clinical trials for the treatment of anemia. Other companies, such as Alnylam Pharmaceuticals and Ionis Pharmaceuticals, are developing nucleic acid-based therapies that target hepcidin and other iron-related proteins.
In addition to therapeutic applications, hngal muteins and nucleic acid encoding such also have potential diagnostic applications. Hngal has been shown to be a biomarker for kidney injury and sepsis, and its levels in the blood can be used to monitor these conditions. By developing hngal-based diagnostic tests, companies can tap into the growing market for point-of-care diagnostics and personalized medicine.
In conclusion, the market for hngal muteins that bind hepcidin and nucleic acid encoding such is a rapidly growing field with potential applications in the treatment and diagnosis of iron-related disorders. As more research is conducted and new therapies are developed, this market is expected to continue to expand and provide new opportunities for companies and investors.
The Pieris Pharmaceuticals GmbH invention works as follows
The present invention is directed to novel therapeutic or diagnostic proteins that bind specifically against Hepcidin. These proteins are preferably muteins from a lipocalin-like protein. The invention also relates nucleic acids encoding these proteins, and methods for generating and using such nucleic acids and proteins. The invention is also directed at pharmaceutical or diagnostic compositions that include such lipocalin protein, as well as uses of the proteins.
Background for Human neutrophil gelatinase-associated lipocalin (hngal) muteins that bind hepcidin and nucleic acid encoding such
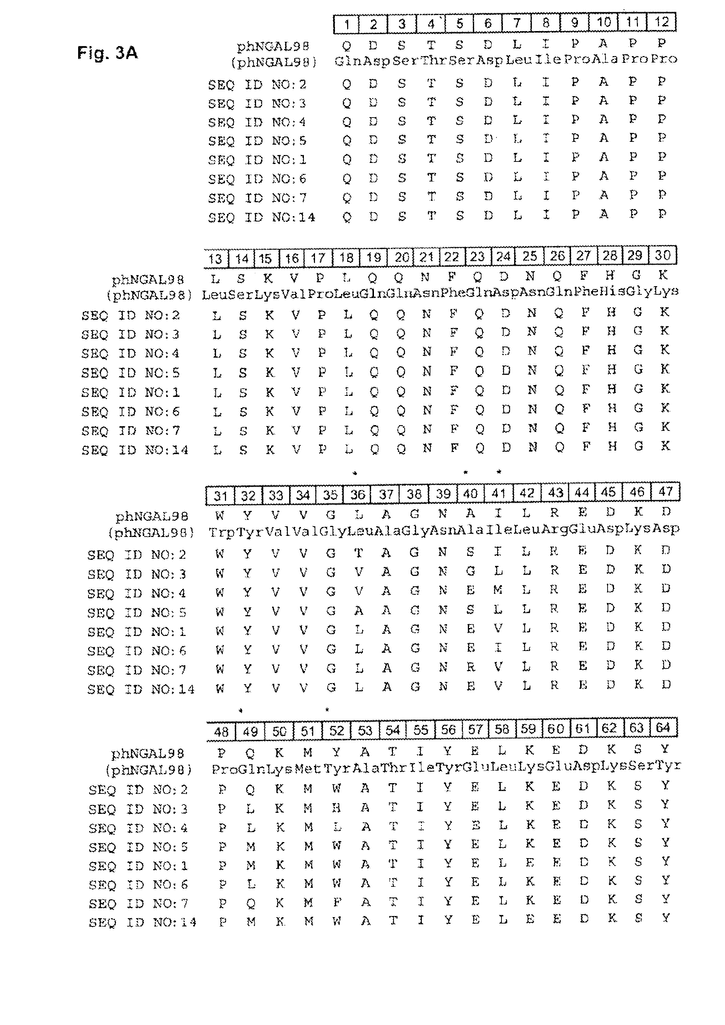
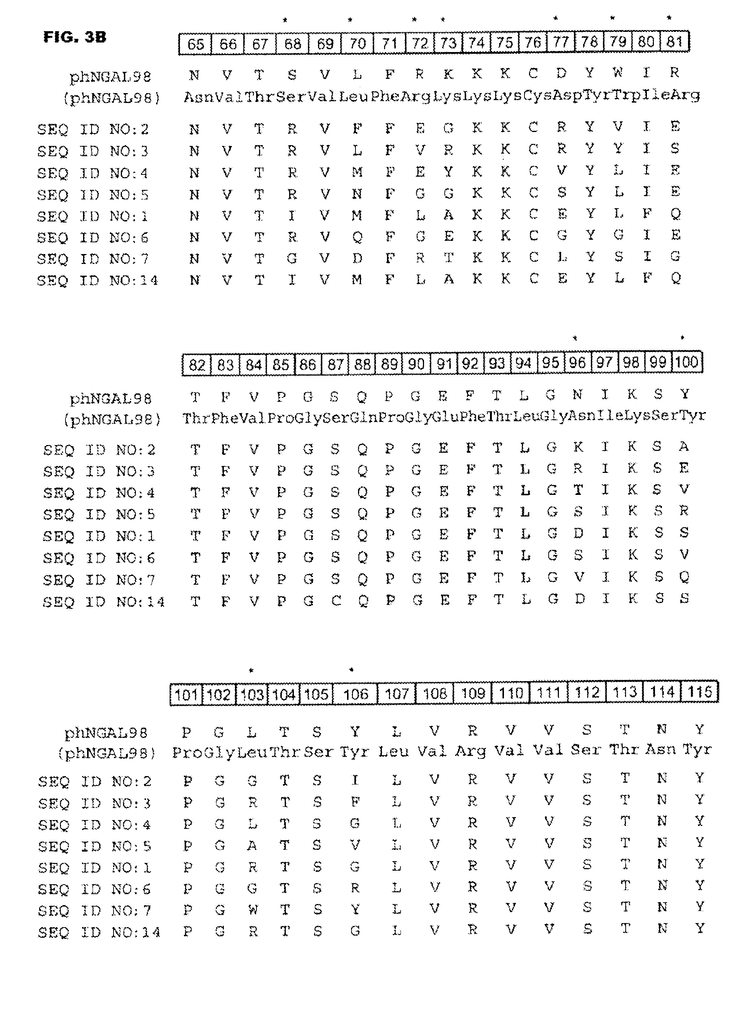
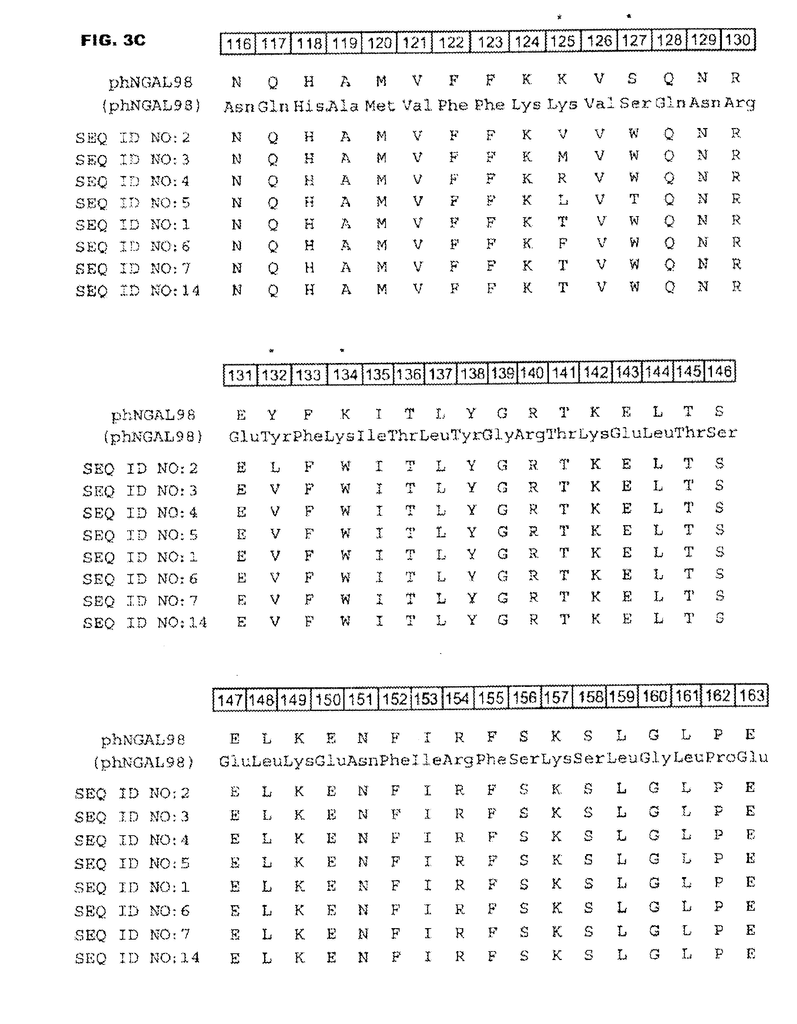
DESCRIPTION of FIGURES
EXAMPLE 1
Construction a Mutant Lcn2 Virus Display Library
EXAMPLE 2
Procurement Of Soluble Hepcidin Peptides
EXAMPLE 3
Generation of a Library With 10 Billion Independent NGAL Muteins
EXAMPLE 4
Phagemid presentation and selection of NGAL Muteins that have affinity for human Hepcidin”.
EXAMPLE 5
High-Throughput ELISA Screening for Identification of hHepcidin Specific Muteins
EXAMPLE 6
Production of Hepcidin Binding Muteins
EXAMPLE 7
Affinity measurement using ELISA techniques
EXAMPLE 8
Affinity Measurement Using Surface-Plasmon-Resonance (SPR)
EXAMPLE 9
Cell Based Assay for Hepcidin Induced Internalization of Ferroportin and Degradation
EXAMPLE 10
Anti-Hepcidin Lipocalin Muteins Neutralize Human Hepcidin in mice
Click here to view the patent on Google Patents.