Invented by Ercole Rao, Vincent Mikol, Danxi Li, Jochen Kruip, Matthew Davison, Sanofi SA
Asthma is a chronic respiratory disease that affects millions of people worldwide. It is characterized by inflammation and narrowing of the airways, leading to symptoms such as wheezing, coughing, and shortness of breath. While there are several treatments available for asthma, including inhalers and oral medications, researchers are constantly searching for new and more effective therapies.
One promising avenue of research is the use of antibodies that bind to interleukin-4 (IL-4) or interleukin-13 (IL-13), two cytokines that play a key role in the development of asthma. IL-4 and IL-13 are produced by immune cells and promote inflammation in the airways, leading to the symptoms of asthma.
Antibodies that bind to IL-4 or IL-13 can block their activity, reducing inflammation and improving asthma symptoms. Several companies are currently developing these antibodies as potential treatments for asthma.
One such antibody is dupilumab, which was approved by the FDA in 2018 for the treatment of moderate-to-severe asthma. Dupilumab targets both IL-4 and IL-13 and has been shown to reduce asthma exacerbations and improve lung function in clinical trials.
Another antibody in development is tralokinumab, which specifically targets IL-13. In a phase II clinical trial, tralokinumab reduced asthma exacerbations and improved lung function in patients with severe asthma.
The market for antibodies that bind IL-4 or IL-13 is expected to grow in the coming years as more therapies are developed and approved for use. According to a report by Grand View Research, the global asthma therapeutics market is expected to reach $23.5 billion by 2025, driven in part by the development of new biologic therapies such as IL-4 and IL-13 antibodies.
While these antibodies show promise as treatments for asthma, they are not without their limitations. They are expensive and must be administered via injection, which may be inconvenient for some patients. Additionally, they may not be effective for all patients with asthma, and more research is needed to identify which patients are most likely to benefit from these therapies.
In conclusion, antibodies that bind IL-4 or IL-13 represent a promising new avenue of research for the treatment of asthma. While there are still challenges to overcome, these therapies have the potential to improve the lives of millions of people living with asthma. As research continues, we may see even more effective and targeted therapies developed in the future.
The Sanofi SA invention works as follows
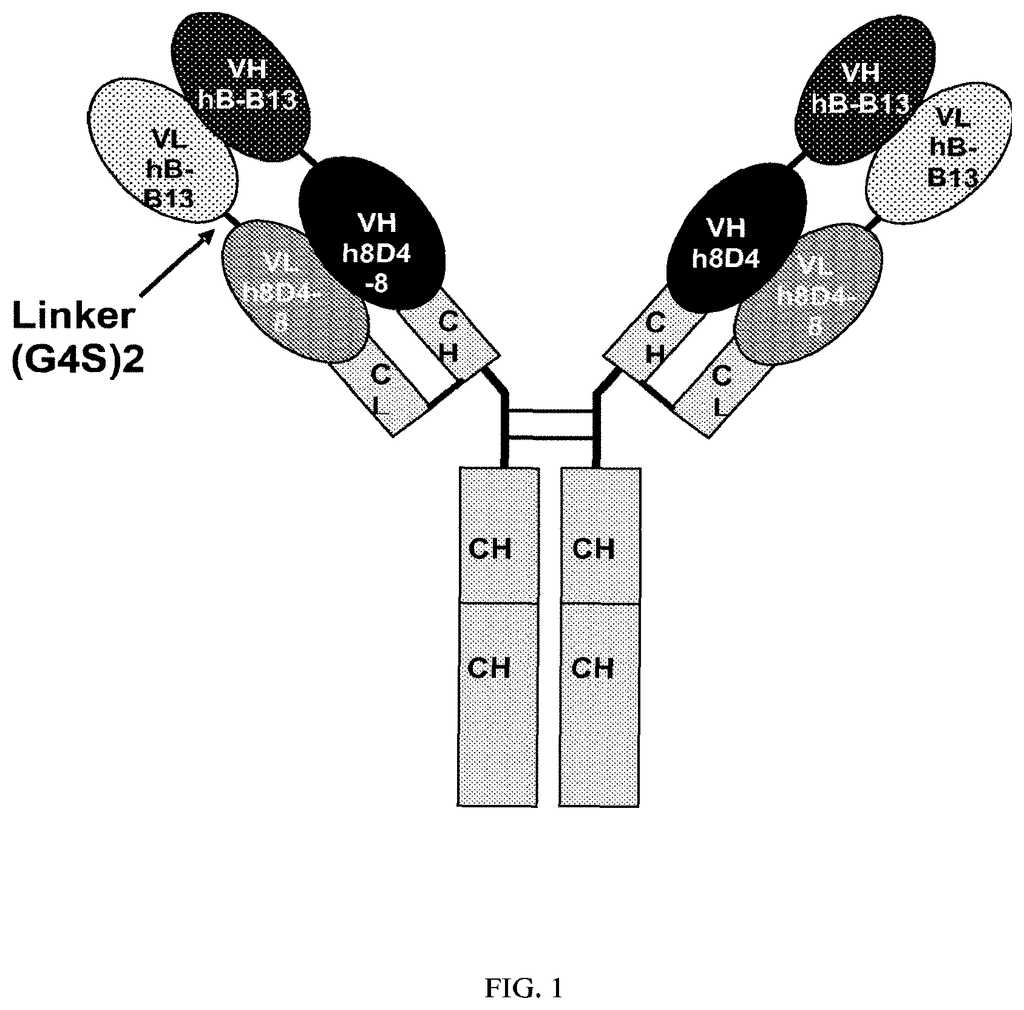
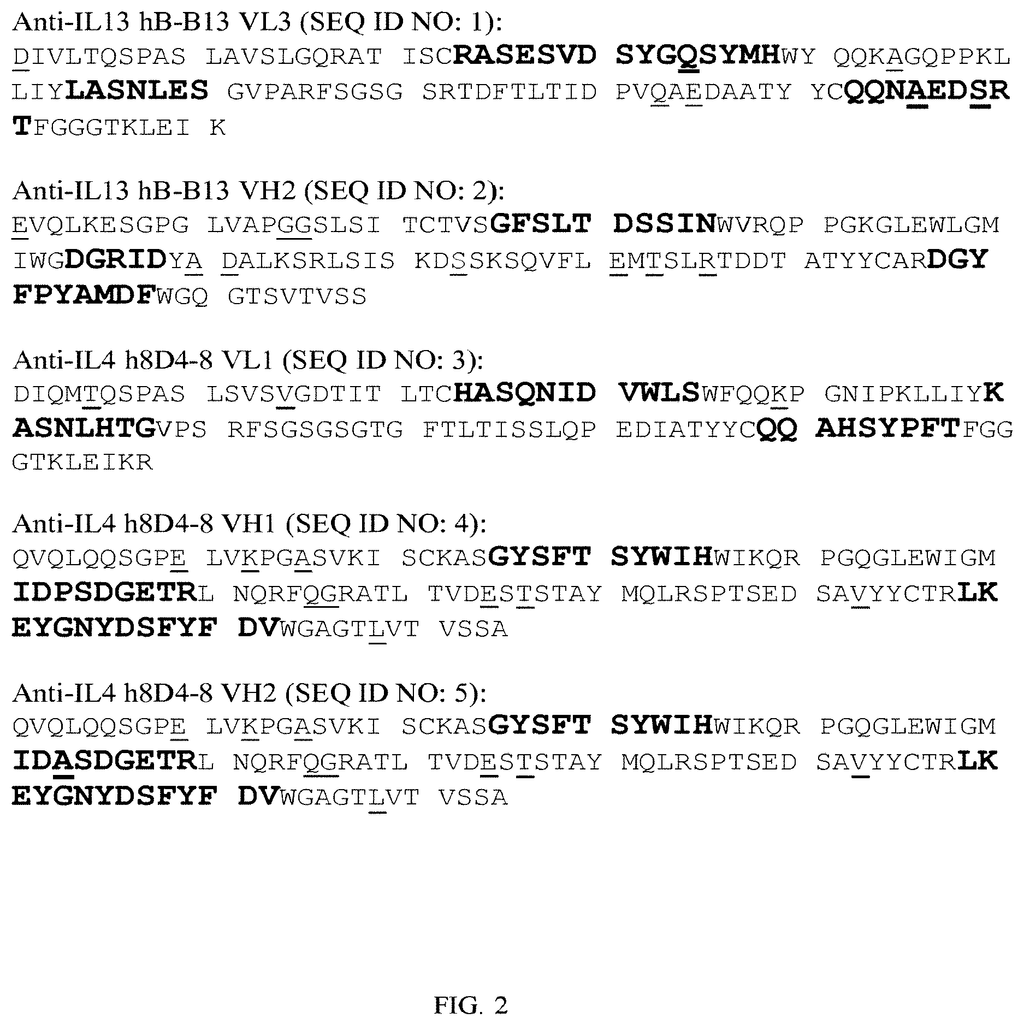
The present invention relates both to humanized anti-IL-4, IL-13 antibodies as well as novel bispecific antibodies that bind specifically to IL-4 or IL-13. The antibodies can also be used to treat or prevent IL-4 or IL-13 mediated disorders or diseases, such as allergic asthma or dermatitis.
Background for Antibodies that bind IL-4 or IL-13, and their use
Interleukin-4 is a pleiotropic cytokine with a wide range of biological effects. It affects lymphoid T and B cells as well as monocytes, fibroblasts, and endothelial and epithelial cell types. IL-4, for example, stimulates proliferation of IL-2 and IL-3 dependent cell lines. It also induces expression of class II Major Histocompatibility Complex molecules on resting human B cells and increases the secretion IgG4 or IgE. IL-4 is linked to a Th2-type of immune response. It is produced and promoted by Th2 cells. IL-4 is implicated in a variety of diseases, including allergy and asthma.
IL-13 has been recently identified” (Minty A. et. al. Nature 1993, 362, 248, 250, and McKenzie A. N. et. al. Proc. Natl. Acad. Sci. “U.S.A. 1993, 90, 3735-3739 (cytokine of 112 amino acids secreted after activation by activated T lymphocytes as well as B lymphocytes and mastocytes.
IL-13 is a cytokine that shares many biological properties with IL-4. Its actions are similar to those of IL-4 in B cells (Defrance T. et.al., J. Exp. Med., 1994, 179, 135-143, Punnonen, J. et al., Proc. Natl. Acad. Sci. (USA), 1993, 90, 3730-3734, Fior, R. et al., Eur. Cytokine Network, 1994, 5, 593-600), the monocytes (Muzio, M. R. F. et al., Blood, 1994, 83, 1738-1743, De Waal Malefyt, R. et al., J. Immunol, 1993, 151, 6370-6381, Doyle, A. et al., Eur. J. Immunol. 1994, 24, 1441-1445, Montaner, L. J. et al., J. Exp. Med., 1993, 178, 743-747, Sozzani, P. et al., J. Biol. Chem. 1995, 270 5084-5088). 1994, 343, 32-36). Contrary to IL-4 it has no specific effect on T cells that are resting or active (Zurawuki G. et.al., Immunol. Today, 1994, 15, 19-26).
Various biological activities of IL-13 on the monocytes/macrophages, the B lymphocytes and certain haematopoietic precursors have been described in detail by A. J. Minty as well as in review articles on IL-13. This cytokine also has a pleiotropic impact on other cell types, according to several data. The non-haematopoietic cell types that are directly affected by IL-13 include endothelial, microglial, keratinocytes, kidney and colon cancers.
The identification of a membrane receptor is one of the steps in analyzing the signal sent by a biological molecular within a cell. Research studies on the IL-13-receptor have revealed that IL-13, IL-4, and other molecules share a receptor or, at least, some components of a receptor complex. Med., 1993, 178, 2213-2218, Vita, N. et al., Biol. Chem., 1995, 270, 3512-3517, Lefort, S. et al., Febs Lett., 1995, 366, 122-126). The receptor can be found on the surface of different cell types. Its number varies depending on the cell type. A. J. Minty has indicated the comparative distribution between IL-13 and IL-4 IL-4 receptors (Interleukin-13 For Cytokines In Health and Disease). Eds D. G. Remick, J. S. Frie and Marcel Decker N.Y. 1996).
The cell surface receptors or receptor complexes bind IL-4 with different affinities. The principal components of receptors or receptor complexes which bind IL-4 or IL-13 include IL-4R, IL-13R and IL-13R2. These chains are expressed as monomers and heterodimers on the cell surface, IL-4R/IL-13R/?1 (Type II IL-4R), or IL-4R/?c/IL-13R/?1 (Type I IL-4R). IL-4R? IL-4R monomer and IL-4R?/?c homodimer bind IL-4 but not IL-13. IL-13R1 and IL-13R2 monomers bind IL-13 but not IL-4. IL-4R?/IL-13R?1 heterodimer binds both IL-4 and IL-13 (Murata et al., Int. J. Hematol., 1999, 69, 13-20).
Th2-type immune response promotes antibody production, humoral immunity and is elaborated to combat extracellular pathogens. Th2 cells mediate Ig production and humoral immunity. They produce IL-4 and IL-5. Th2-type responses are characterized as the production of specific cytokines, e.g. IL-4 and IL-13, and antibodies, IgE and IgG4. They are characteristic of allergic reactions and can result in symptoms such as watery eyes, asthmatic symptoms and airway inflammation.
Both IL-4 (and IL-13) are important cytokines for treating many diseases. This includes asthma. 5, 161-166). IL-4 inhibits autoimmune diseases and IL-4, IL-13 and IL-13 both have the ability to boost anti-tumor immunity responses. Inhibitors of both cytokines, which are involved in allergic diseases’ pathogenesis, could have therapeutic benefits.
Accordingly there is a need for improved agents which inhibit IL-4 or IL-13 and agents that can inhibit both IL-4 as well as IL-13.
The present invention provides novel humanized bispecific and monoclonal antibodies, fragments, and derivatives of these antibodies that bind specifically to IL-4 or IL-13. The anti-IL-4 or IL-13 monoclonal and bispecific antibodies and fragments can be altered in order to prevent the formation of intrachain disulfide bonds. This results in a molecule which is stable during manufacturing and in vivo. The antibodies of the invention neutralize IL-4 or IL-13 activity when used in the biological assays.
The invention contains the amino acid sequences for the heavy and light chains of antibodies that are variable and the nucleic acids sequences that correspond to them.
The present invention can also be embodied in the form of cell lines or vectors that harbor the antibody sequences.
Another embodiment of the invention involves the use of antibodies to prepare a pharmaceutical composition that is used for treating diseases and disorders related to IL-4 or IL-13 metabolism and function. The present invention is particularly suited to treating cancer, autoimmune disorders, and diseases characterized or caused by inflammation such as allergic asthma, dermatitis, and rheumatoid arthritis.
The following Detailed Description, as well as the Figures, will make it clear that there are additional features and benefits.
This invention is not restricted to the specific methodologies, protocols, cell-lines, vectors or reagents that are described in this document, because they can be varied without compromising the spirit and scope. The terminology used in this document is only to illustrate particular embodiments and not to limit the scope. All technical and scientific terminology and acronyms are used in this document with the same meaning as understood by a person of ordinary skill. “Any method or material equivalent to that described herein may be used for the practice of this invention. Only exemplary methods, materials, and devices are described.
All patents and publications cited herein are incorporated in their entirety by reference to describe and disclose the proteins, enzymes vectors host cells, and methods reported therein which could be used in conjunction with the present invention. The present disclosure is not intended to imply that the invention does not have the right to predate it by virtue of prior discovery.
The following definitions are not intended to limit the artisan’s ability to make and use the IL-4 or IL-13-related methods and products.
?Interleukin-4? {(IL-4) relates to the naturally occurring, or endogenous mammalian IL-4 proteins and to proteins having an amino acid sequence which is the same as that of a naturally occurring or endogenous corresponding mammalian IL-4 protein (IL-4) relates primarily to naturally occurring, endogenous mammalian IL-4 and proteins with an amino acid pattern that is identical to a mammalian IL-4 naturally occurring protein (e.g. recombinant or synthetic proteins produced by synthetic organic chemistry). As defined in this document, IL-4 includes the mature IL-4, polymorphic and allelic variants and other isoforms as well as modified and unmodified versions of these (e.g. lipidated or glycosylated). Endogenous or naturally occurring IL-4 is composed of wild-type proteins, such as mature IL-4 and polymorphic or allelelic variants, and other mutant and isoform forms that occur in mammals. These proteins can be isolated or recovered from a source that naturally produces IL-4. These proteins, and proteins with the same amino-acid sequence as a naturally occuring or endogenous corresponding IL-4 are referred by the name of corresponding mammal. If the corresponding mammal in this case is a person, then the protein will be designated as a IL-4 for humans. Several mutant IL-4 protein are known to the art. For example, those disclosed in WO03/038041.
?Interleukin-13? (IL-13), refers to mammalian IL-13 naturally occurring proteins or proteins with an amino acid pattern that is identical to a mammalian IL-13 naturally occurring protein or protein produced by synthetic organic chemistry. As defined in this document, IL-13 includes mature IL-13, polymorphic and allelic variants and other isoforms (e.g. produced by alternative splicing, or other cellular processes), as well as modified or unmodified versions of these (e.g. Hpidated, or glycosylated). Endogenous IL-13 includes wild-type proteins, such as mature IL-13 and polymorphic or allelelic variants, and other isoforms or mutant forms that occur naturally in mammals. As used herein, IL-13 includes the human IL-13 variation in which Arg is replaced by Gin at position 110 (position 110 of adult human IL-13 corresponds with position 130 of the precursor) which is linked to asthma (atopic or nonatopic asthma), and other variants. (Heinzmann et al, Hum Mol Genet. 9:549-559 (2000).) These proteins can be isolated or recovered from a source that naturally produces IL-13. The name of the mammal corresponding to the protein or proteins with the same amino acids sequence as a naturally occuring or endogenous corresponding IL-13 is used for these proteins. If the corresponding mammal in this case is a person, then the protein will be designated as a “human IL-13”. Several mutant IL-13 protein are known to the art. For example, those disclosed in WO03/035847.
The phrase “substantially similar” can be interpreted as an antibody chain that exhibits at least 70%, 80%, 90% or 95% sequence identity to the reference polypeptide sequence. The term “substantially identical” can be used to describe an antibody polypeptide chain that exhibits at least 70% 80% 90% 95% or greater sequence identity with the reference polypeptide. A nucleic sequence can be defined as a sequence of nucleotides that exhibits at least 85%, 90% or 95% sequence identity with the reference nucleic sequence.
The terms, ?identity? “The terms?identity? or?homology’ can refer to the percentage of nucleotide bases or amino acid residues in a candidate sequence that are identical with the residues of a corresponding sequence to which it is compared, after aligning the two and introducing gaps, if necessary, to achieve maximum percent identity for the entire sequence. The percentage of residues that are identical to the residues of another sequence is called?homology’. This can be calculated by aligning the sequences, inserting gaps if needed, and then calculating the percent identity of the entire sequence. The N-terminal and C-terminal insertions or extensions are not to be considered as reducing homology or identity. Alignment methods and computer programs are well-known and available. “Sequence analysis software can be used to measure sequence identity.
Click here to view the patent on Google Patents.