Invented by Richard J. Gregory, Donna Armentano, Larry A. Couture, Alan E. Smith, Genzyme Corp
The market for adenoviral vectors for gene therapy containing deletions within the adenoviral genome is expected to grow significantly in the coming years. This is due to the increasing demand for safer and more effective gene therapies, as well as the growing number of clinical trials using these vectors.
One of the main advantages of adenoviral vectors containing deletions within the adenoviral genome is their reduced immunogenicity. By deleting certain genes within the adenoviral genome, researchers can reduce the immune response to the vector, which can improve the safety and efficacy of gene therapy. Additionally, these vectors can be engineered to target specific cell types, which can improve the specificity of gene therapy and reduce off-target effects.
Several companies are currently developing adenoviral vectors for gene therapy containing deletions within the adenoviral genome. For example, Oxford BioMedica is developing a vector called LentiVector, which is based on a lentiviral backbone and contains deletions within the adenoviral genome. This vector has been used in several clinical trials for the treatment of genetic disorders, including X-linked chronic granulomatous disease and Wiskott-Aldrich syndrome.
Another company, Genprex, is developing a vector called Oncoprex, which is designed to deliver a tumor suppressor gene to cancer cells. This vector contains deletions within the adenoviral genome to reduce its immunogenicity and improve its safety profile.
The market for adenoviral vectors for gene therapy containing deletions within the adenoviral genome is expected to grow significantly in the coming years. As more clinical trials are conducted and more gene therapies are approved, the demand for safer and more effective vectors will continue to increase. With their reduced immunogenicity and ability to target specific cell types, adenoviral vectors containing deletions within the adenoviral genome are poised to play an important role in the future of gene therapy.
The Genzyme Corp invention works as follows
Adenoviral Vectors that contain deletions of early regions and/or genes late provide efficient delivery of foreign nucleic acid of interest to the patients. These vectors are used in particular for the treatment of patients with cystic fibrosis. The PAV vectors also provide a second-generation of adenoviral vectors that include the 5′ ITR, the packaging signal, and the E1A promoter. Other adenoviral Vectors have a deletion in the E1 region, or a deletion in E4, but they retain orf3 and orf6. They can also retain or delete E3 regions.
Background for Adenoviral Vectors for Gene Therapy containing deletions within the adenoviral Genome
Boat, T. F. et. al. “Cystic Fibrosis is the most common genetic fatal disease in humans.” “Cystic Fibrosis (CF) is the most common fatal genetic disease in humans” (Boat, T. F. et. al. eds., McGraw-Hill, N.Y. (1989)). In the United States, approximately one out of every 2,500 babies is born with CF. There are currently approximately 30,000 CF sufferers in the United States. The median age for survival is 26 years despite the current standard treatment. The pulmonary airways are the main cause of mortality and morbidity. The first sign of lung disease can be a persistent cough. This is followed by dyspnea. The sputum is tenacious and becomes purulent due to the colonization with Staphylococcus, then Pseudomonas. Current therapy can partially treat chronic bronchitis or bronchiectasis, but there are increasingly frequent exacerbations. The patient’s ability to do anything becomes more and more limited as the disease advances. The end-stage of lung disease is marked by hypoxemia and pulmonary hypertension.
The upper airways and sinuses of the nose are also affected by CF. Chronic sinusitis is common in most CF patients. Nasal polyps are found in 15 to 20% of patients by the age of 20. Infants can also suffer from meconium ileus. The majority of CF patients have exocrine pancreatic dysfunction, which causes symptoms of malabsorption. “Females have a decreased fertility and males are infertile almost universally.
Based on genetic and molecular analysis, a gene related to CF has been isolated from 21 individual cDNA clones, and its predicted protein product (Kerem, B. S. et. al. (1989) Science 245:1073-1080; Riordan, J. R. et al. (1989) Science 245:1066-1073; Rommens, J. M. et al. (1989) Science 245, 1059-1065). U.S. Ser. No. No. 07/488 307 describes the construction and expression of the continuous strand of the gene, as well as the confirmation that mutations in the gene cause CF. (See also Gregory R. J. and al. (1990) Nature 347:382-386; Rich, D. P. et al. Nature 347, 358-362 (1990). The patent application for the co-pending patent also discloses experiments that show that proteins produced from the wild type but not a mutation version of the cDNA complemented CF’s defect in the cAMP regulated channel.
The cystic fibrosis conductance regulator is the protein product of the CF-associated gene (Riordan J. R. et.al. (1989) Science 245, 1066-1073. CFTR, a protein with approximately 1480 amino acid residues, is composed of two repeating elements. Each element consists of six transmembrane segment and a nucleotide-binding domain. The R-domain, which is a large polar domain containing many potential phosphorylation spots, separates the two repeats. According to its predicted domain structure CFTR belongs to a group of proteins that includes bovine adenyl cyclase (BAC), yeast STE6 and several bacterial amino-acid transport proteins. (Riordan J. R. et. al. (1989) Science 245:1066-1073; Hyde, S. C. et al. Nature 346, 362-365 (1990). The proteins in this group are involved in the pumping of molecules into or out from cells.
CFTR is thought to regulate the outward flow from epithelial cell anions in response to phosphorylation of cyclic AMP dependent protein kinase (Riordan J. R. et. al. (1989) Science 245:1066-1073; Welsh, 1986; Frizzell, R. A. et al. Welsh, M. J., and Liedtke C. M. (1986), Nature 322:467, Li, M., et al. (1988) Nature 331:358-360; Hwang, T-C. et al. (1989) Science 244:1351-1353).
Sequence Analysis of the CFTR Gene of CF Chromosomes has revealed a number of mutations” (Cutting, G. R. et. al. (1990) Nature 346:366-369; Dean, M. et al. Dean, M. et. al. (1989) Science 245:1073-1080; Kerem, B-S. et al. (1990) Proc. Natl. Acad, Sci. USA 87:8447-8451). According to population studies, the most common CF-related mutation is a deletion of 3 nucleotides at position 508 in the CFTR amino acids sequence (AF508). This mutation accounts for approximately 70% of all cystic fibrosis cases. This mutation causes an epithelial chloride channel not to respond to the cAMP signal (Frizzell, R.A. et. al. (1986) Science 233, 558-560. Welsh, M.J. (1986) Science 232:1648-1650.; Li, M. et al. Nature 331:358-360 (1988); Quinton, P. M. Chem. 35:726-730). This imbalance leads to a fluid and ion transport imbalance in airway cells. This is believed to cause abnormal mucus production, which leads to pulmonary infection.
Studies on biosynthesis” (Cheng, S. H. (1990) Cell 63:827-834; Gregory, R. J. et al. (1991) Mol. Cell Biol. Cell Biol. (1992) J. Cell Biol. Cell Biol. (1991) Mol. Cell Biol. 11:3886-3893). These conclusions are in line with previous functional studies that failed to detect cAMP stimulated C1-channels in cells expressing CFTR AF508(Rich, D. P. et.al. (1990) Nature 347:358-363; Anderson, M. P. et al. (1991) Science, 251: 679-682).
Boat, T. F. et. al., “Today, the primary goals of treatment for CF are to control infection and promote mucus clearing, as well as improve nutrition.” “To date, the primary objectives of treatment for CF have been to control infection, promote mucus clearance, and improve nutrition (Boat T. F. et.al. McGraw-Hill New York, 1989, eds. The mainstays of treatment are intensive antibiotic use, chest percussion and postural drainage. As the disease advances, hospitalizations become more frequent. The nutritional regimen includes pancreatics enzymes and fat soluble vitamins. Sometimes, bronchodilators may be used. Although corticosteroids are used to reduce inflammation and may have adverse effects, their effectiveness is not guaranteed. Marshall, S., et. al. (1990) Chest 98:1488). Chest 98, 1488 (1990).
Most efforts to develop new treatments for CF are focused on the pulmonary problems. CF mucus contains a large amount of DNA derived from lysed neutrophils. One approach was to develop recombinant DNase. (1990) Proc. Natl. Sci. Acad USA (87:9188). Initial reports suggest that aerosolized enzymatic solution may reduce the viscosity mucus. This could help clear the airways and reduce infections. Protease inhibitors were tested to try and limit the damage caused by excess neutrophil-derived elastase. McElvaney, N. et al. (1991) The Lancet 337:392). The Lancet, 337:392, 1991. A second approach would be to use agents that inhibit the action of neutrophil-derived oxidants. “Although biochemical parameters were successfully measured, long-term benefits of these treatments had not been established.
Other investigators, using a different rationale have tried to reverse the abnormally reduced chloride secretion in CF and increased sodium intake in CF airways. Boat, T. F. and colleagues suggest that a defect in electrolyte transportation by the airway epithelial alters the composition of respiratory secretions and muck. In The Metabolic Basis of Hereditary Diseases, (Scriver C. R. and others. McGraw-Hill New York (1989); Quinton P. M. (1990), FASEB Journal 4:2709-2717). Pharmacological treatments that correct the abnormalities of electrolyte transportation could be helpful. Aerosolized versions are being tested for amiloride, a diuretic which inhibits sodium channels and inhibits sodium absorption. Initial results show that the drug is safe, but suggest that it may slow down the progression of the disease, as measured through lung function tests. (1990) N. Eng. J Med. 322: 1189-1194; App, E.(1990) Am. Rev. Respir. Dis. 141:605. Nucleotides such as ATP and UTP stimulate purinergic membrane receptors. They open a different class of chloride channels than CFTR. In vitro experiments indicate that ATP or UTP can increase chloride secretion. M. et al. (1991) N. Eng. J. Med. 325:533). “Preliminary trials are being conducted to test nucleotides’ ability to stimulate secretion and correct electrolyte transportation abnormalities in vivo.
Despite advances in therapy, Cystic Fibrosis is still a deadly disease and there is no treatment that addresses the fundamental defect. Two general approaches could be feasible. There are two general approaches: 1) protein substitution therapy, which delivers the wild-type protein to patients in order to augment the defective protein; and 2) gene replacement therapy, which delivers wild-type copies of the CF gene. “Since the most serious manifestations of CF are pulmonary complications and epithelial cell of the upper airways is the appropriate target cell for therapy.
The feasibility for gene therapy was established by introducing a cDNA from a wild-type CF patient into epithelial cell and demonstrating the complementation of a hallmark defect in chloride ion transportation (Rich, D. P. et. al. Nature 347, 358-363 (1990). The initial work was done on cells in tissue cultures, but subsequent research has shown that defective adenoviruses can be used to deliver the gene into the airways of animals (Rosenfeld (1992), Cell 68:143-155). The safety and effectiveness of using defective viruses must be proven.
In general the present invention is a vector for transferring genetic material (e.g. DNA or RNA), to cells in vivo. The vectors used in preferred embodiments are adenovirus based. Adenovirus vectors are advantageous for gene therapy because they are relatively safe, can be modified to encode the desired product, and are also inactivated from the perspective of their ability of replication in a normal viral life cycle. Adenoviruses are also naturally attracted to airway epithelia. Adenovirus vectors are therefore preferred for respiratory gene therapies such as cystic fibrosis gene therapy.
In one embodiment, an adenovirus-2 serotype genome has been modified to remove the E1a-E1b regions, which are responsible for the early stages of viral reproduction, and replace them with genetic material (e.g. DNA encoding a cystic fibrosis regulator transmembrane protein).
In another embodiment, the adenovirus based therapy vector is pseudo-adenoviruses (PAVs). PAVs do not contain any potentially harmful viral gene, they can be produced at high levels and retain the same tropism as the parent adenovirus in both dividing and nondividing target cells. PAVs contain adenovirus terminal inverted repeats, and minimal sequences from a wild type adenovirus 2 genome required for efficient replication by a helper Virus and genetic material. The PAV is preferably adenovirus type 2 sequences.
In another embodiment, the vector based on adenovirus contains the open reading frame 6 (ORE6) from adenovira’s ear region 4, (E4) and is deleted all other E4 Open Reading Frames. This vector may include deletions of the E1 or E3 regions. The adenovirus based gene therapy vector can also contain the ORF3 of the adenoviral E4 and be deleted for the other E4 open-reading frames. Optionally, the vector can also include deletions of E1 or E3 regions. The deletion of E4 non-essential frames increases the cloning capability by about 2 kb, without affecting the viability of virus in cell cultures. Combining deletions of E1 or E3 regions in adenoviruses increases the theoretical insert size of the vectors to 8-9kb.
The invention also relates methods of genetherapy using the vectors disclosed and the genetically engineered cell produced by the method.
BRIEF DISCLOSURE OF THE TABLES & DRAWINGS”.
The tables and figures in the following paragraphs will help you to better understand the invention.
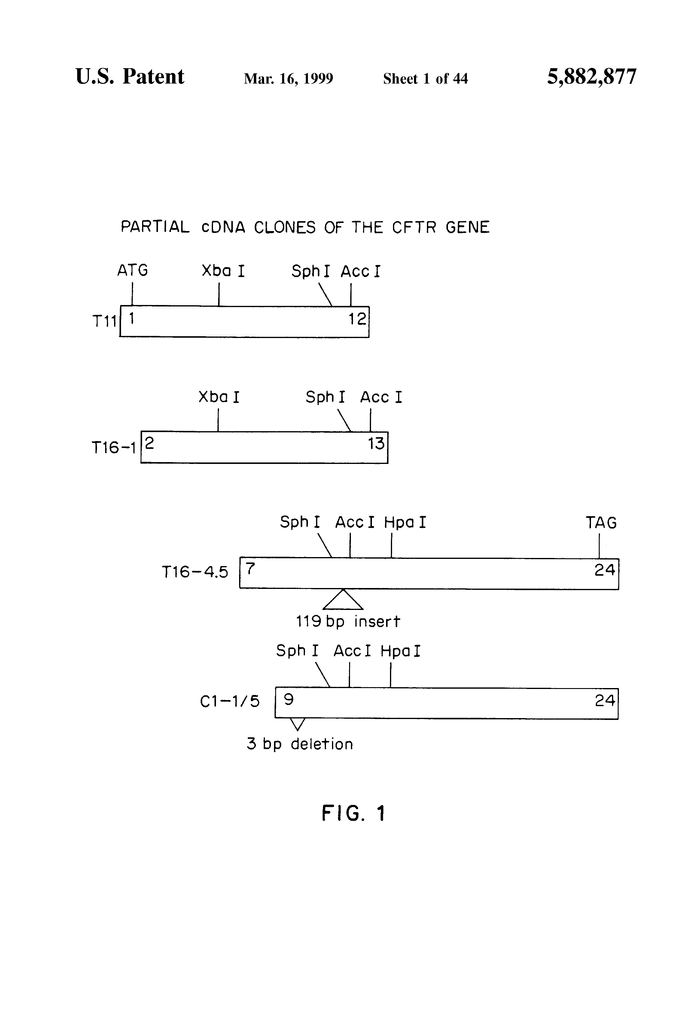
FIG. “FIG.
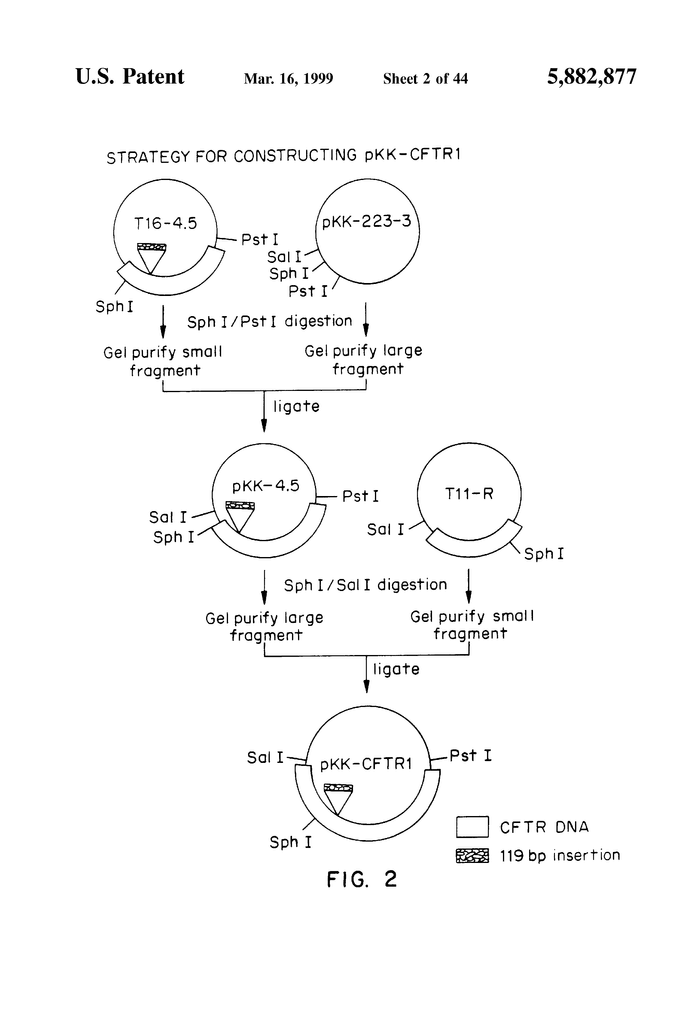
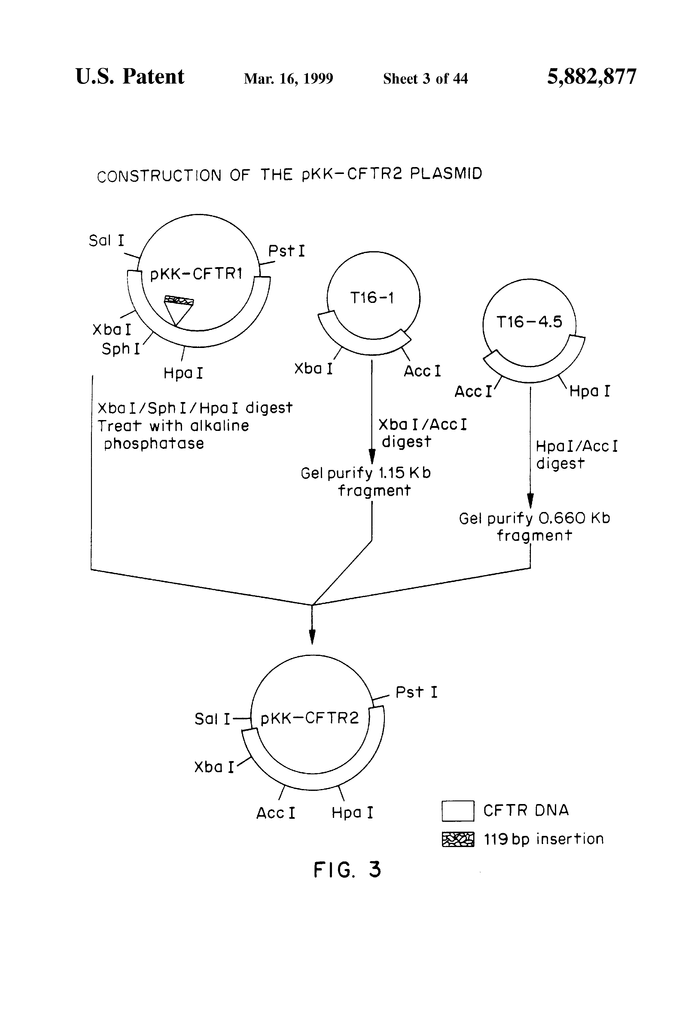
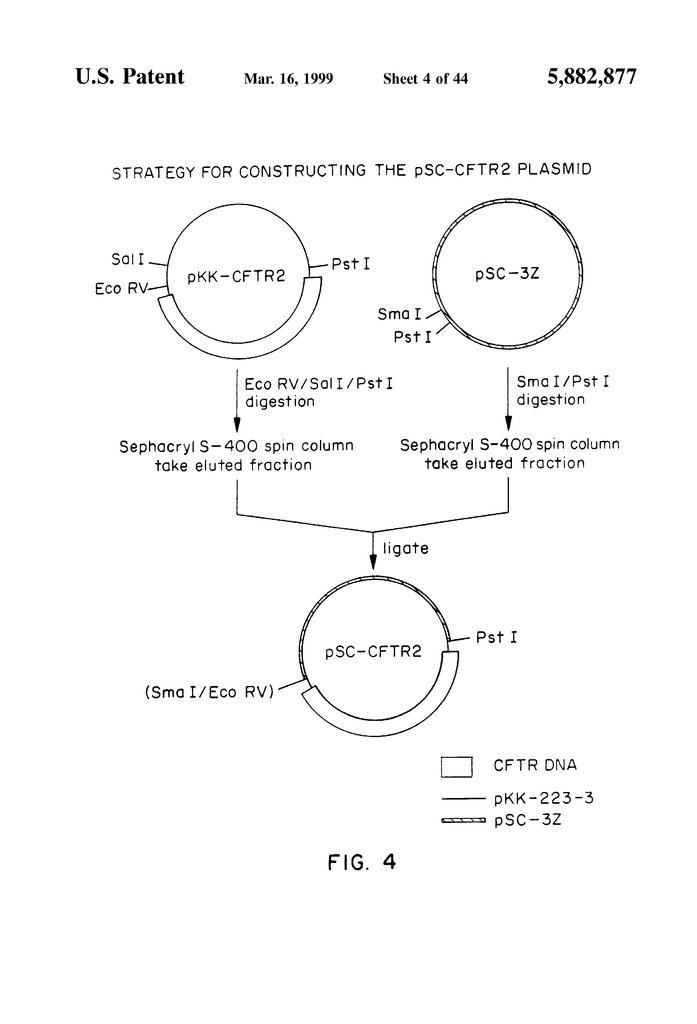
FIG. “FIG.
Click here to view the patent on Google Patents.