Invented by Michael Joseph Weiss, Ryan J Gilliam, Kyle Self, Gal Mariansky, Margarete K Leclerc, Riyaz Mohammed Shipchandler, Jacob Nagar, Fortera Corp
The global market for separation and purification systems is expected to reach USD 28.7 billion by 2025, growing at a CAGR of 6.7% from 2020 to 2025. The market is driven by factors such as the increasing demand for biopharmaceuticals, the growing adoption of green chemistry, and the need for efficient separation and purification processes.
The pharmaceutical industry is one of the major end-users of separation and purification systems. The increasing demand for biopharmaceuticals such as monoclonal antibodies, vaccines, and recombinant proteins has led to the development of advanced separation and purification technologies. Chromatography, filtration, and centrifugation are some of the commonly used techniques in the pharmaceutical industry for separation and purification.
The food and beverage industry is another major end-user of separation and purification systems. The increasing demand for high-quality food products has led to the adoption of advanced separation and purification technologies such as membrane filtration, reverse osmosis, and evaporation. These technologies help to remove impurities and contaminants from food products and improve their shelf life.
The chemical processing industry is also a significant end-user of separation and purification systems. The increasing demand for high-purity chemicals has led to the development of advanced separation and purification technologies such as distillation, crystallization, and extraction. These technologies help to remove impurities and contaminants from chemicals and improve their quality.
In conclusion, the market for systems and methods for separation and purification of products is expected to grow significantly in the coming years. The increasing demand for high-quality products in various industries such as pharmaceuticals, food and beverage, and chemical processing is driving the growth of the market. Advanced separation and purification technologies are essential in these industries as they help to remove impurities and contaminants from raw materials and finished products.
The Fortera Corp invention works as follows
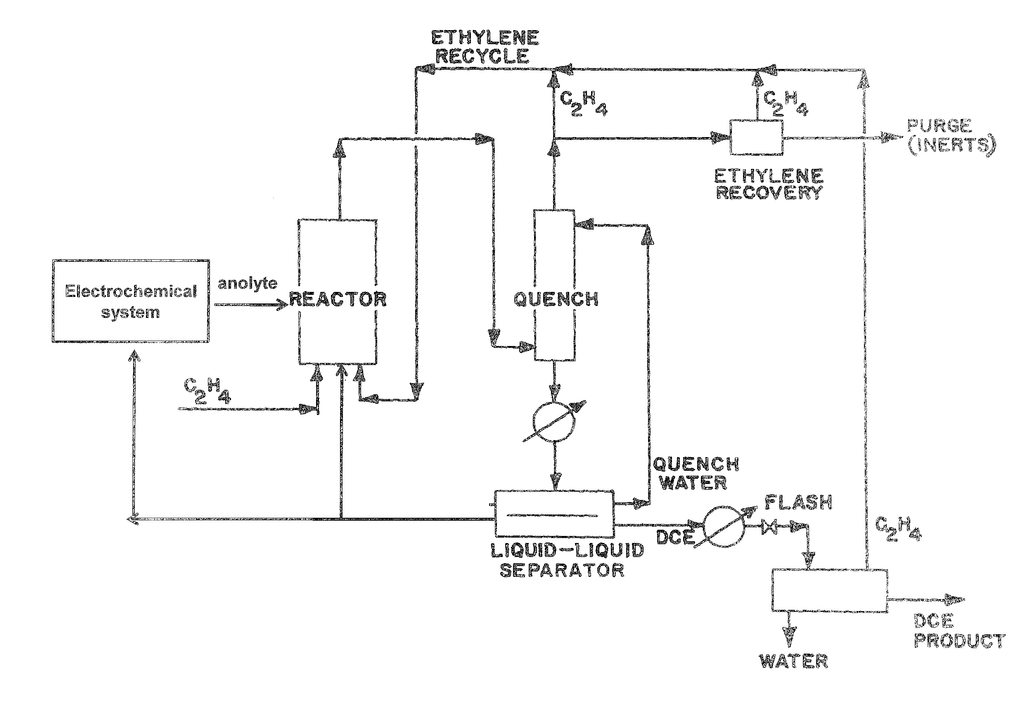
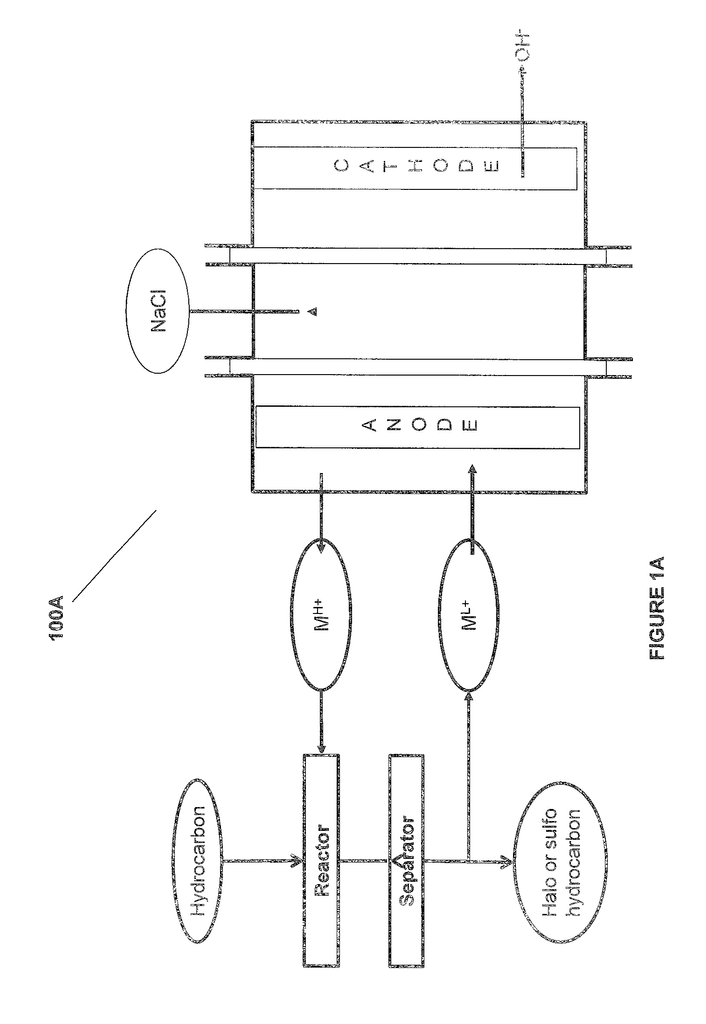
Methods and systems are provided for an electrochemical system including an anode, and a cathode. The anode contacts a metalion to convert it from a lower state of oxidation into a higher state. The metal ion is in a higher oxidation and reacts with an unsaturated or saturated hydrocarbon. The present invention provides for the separation and/or purification both of the metal ions and products in the lower and higher oxidation states.
Background for Systems and Methods for Separation and Purification of Products
In many chemical reactions, caustic soap is required. For example, it may be needed to neutralize an acid, buffer the pH of a liquid, or precipitate a hydroxide that is insoluble from a liquid. Electrochemical systems are one way to produce caustic soda. When producing caustic soda via electrochemical process, such as the chlor-alkali method, large amounts of salt and water can be used.
PVC is the third most widely produced plastic after polyethylene (polypropylene) and polyethylene (polyethylene). It is used widely in construction due to its durability, low cost, and ease of workability. PVC can be produced by polymerizing vinyl chloride monomer, which is made from ethylene Dichloride. “Ethylene Dichloride can be produced by chlorinating ethylene with chlorine gas from the chlor-alkali reaction.
The production of caustic soda and chlorine by electrolysis from aqueous sodium chloride solutions or brines is an electrochemical process that requires high energy consumption. For example, the total energy required to maintain the chlor-alkali process is about 2% of the gross electricity generated in the USA and 1% in Japan. High carbon dioxide emissions from burning fossil fuels may contribute to the high energy consumption. To reduce global warming and environmental pollution, it is necessary to reduce the demand for electrical energy.
The system comprises: “In one aspect there is provided an electrochemical system which includes: an anode-chamber comprising an metal anode in an anode electrode electrolyte containing metal ions, the anode being configured to oxidize metal ions to a higher state of oxidation, and a reactor connected to the chamber and configured for contacting the anode anode comprising the metal metal ions with an unsaturated hydrocarbon or saturated hydrocarbon in order to form
The system comprises an electrochemical system that includes an anode, anode-electrolyte, and an anode, wherein the anode has the ability to oxidize metal ions from a lower to higher oxidation states; a reactor connected to the chamber, and configured to react a hydrocarbon, either unsaturated or saturated, in an aqueous media with the anode electrodelyte containing the metal in the higher state, to produce one or several organic In some embodiments, the separator includes a reaction separation reactor system. Other options include a compression-cooling, liquid-liquid, vapor-liquid, scrubbing, or purification subprocess systems. In certain embodiments and aspects of the above, the reactor can be a reaction separator. In some embodiments the reaction separation reactor can be a reactive distillation or extraction reactor. In some embodiments the liquid-liquid separator system includes a decanter or extractant; the vapor/liquid separator system includes a flash column or a distillation system; the purification system contains a decanter or sorbent; or a column containing an alkali.
In some embodiments, the systems described above, the un-saturated hydrocarbons (such a ethylene), the saturated hydrogens (such a formula III), halogenated hydrocarbons, metal ions, and so on, are all described in detail. All of these have been described in great detail. “In some embodiments, the metal ion in the system is copper, and the unsaturated hydrogen is ethylene. The organic compound is EDC or combinations thereof.
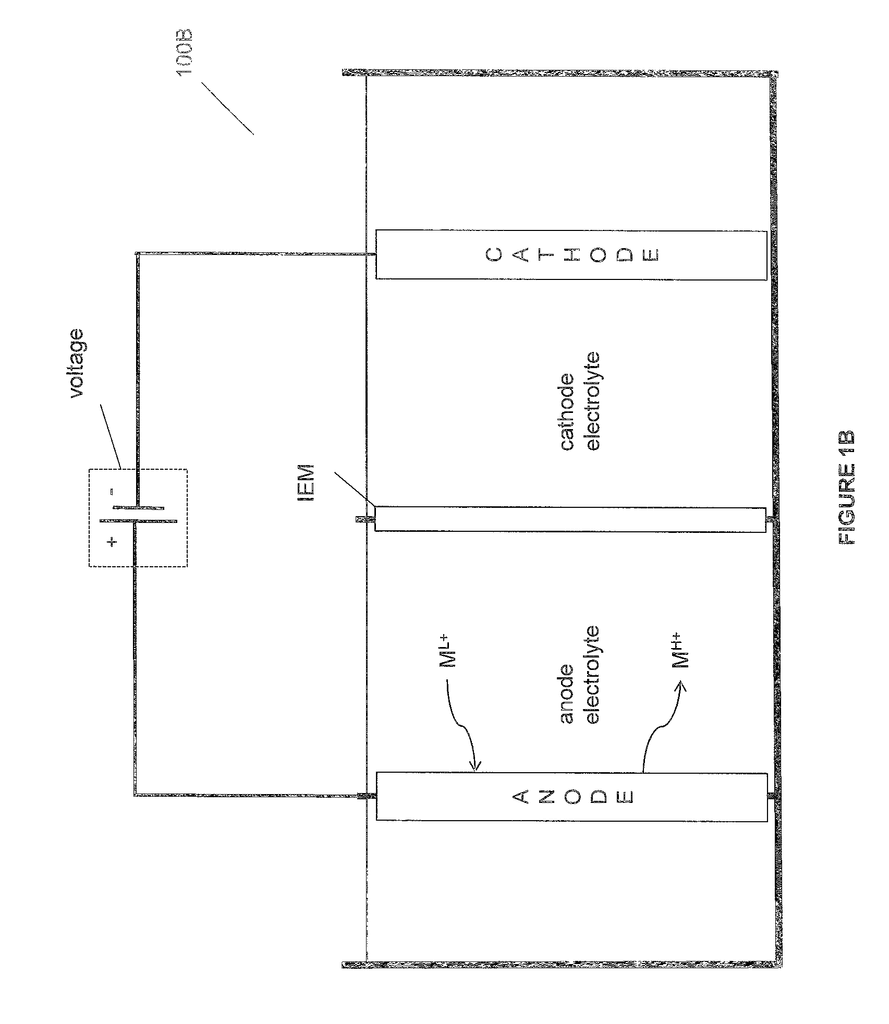
In some embodiments of aforementioned aspects and embodiments, anode from cathode are separated by anion-exchange membranes made of reinforced materials with a thickness between 20-130 mm or 20-75 mm or 50-100 mm. These membranes are described in more detail. In certain embodiments of the above aspect and embodiments the anion-exchange membrane separates the anode from a third electrode electrolyte. In some embodiments, a cation-exchange membrane separates the third electrodelyte and cathode from each other.
In some embodiments the separator comprises a recirculating device operably connected to anode for recirculating the aqueous media containing metal ions in the lower state of oxidation to the anode electrode electrolyte.
In some embodiments the anode can be a diffusion-enhancing anode, such as a porous one. As described herein, the porous anode can be corrugated or flat.
In some embodiments the separator also contains an adsorbent chosen from activated carbon, alumina or activated silica.
In some embodiments the ligand of the anode is also included in the electrolyte. The ligand interacts with the metal ion.
In some embodiments of aforementioned system aspects and embodiments, a cathode that is configured to react oxygen and water in order to produce hydroxide ions. In some embodiments of aforementioned system aspects and embodiments, the hydrogen gas-producing cathode can reduce water to produce hydrogen gas and hydroxideions. In some embodiments of aforementioned system aspects and embodiments the cathode can be a hydrogen producing cathode that is configured to convert an acid such as hydrochloric to hydrogen gas. In some embodiments the system aspect and embodiments are configured with a gas diffusion cathode that reacts hydrochloric and oxygen to produce water.
In some embodiments the anode of the system is configured so that it does not produce a gas.
In some embodiments the aforementioned aspects and embodiments further comprise a precipitator that is configured to contact the cathode electrode electrolyte and divalent cations in order to form a bicarbonate or carbonate product.
In some embodiments the metal ion in the above aspect and embodiments is copper. In some embodiments the unsaturated hydrocarbon in the aforementioned aspects and embodiments is ethylene. In some embodiments of the aforementioned aspect and embodiments, the one or more organic compounds are selected from ethylene dichloride, chloroethanol, dichloroacetaldehyde, trichloroacetaldehyde, and combinations thereof.
In another aspect, a method is provided that comprises contacting an anode and an anode electrode electrolyte, wherein the anode electrode electrolyte contains metal ion, oxidizing the metallic ion at the anode to a higher state of oxidation, reacting in an aqueous media an unsaturated, or saturated, hydrocarbon to form one, or more, organic compounds containing halogenated, or sulfonated, hydrocarbon In some embodiments, the purification and separating step includes a reaction separation process. Other options include compression-cooling, liquid-liquid, vapor-liquid, scrubbing, adsorption and combinations of these. In some embodiments and aspects of the aforementioned, the reacting process includes reaction separation.
In some embodiments in the above aspect and embodiments the reaction separation consists of reactive distillation or reactive extraction.
In some embodiments the liquid-liquid process is decantation or extraction or a combination of both; the vapor/liquid process is flash distillation or distillation or a combination thereof; or the purification process includes decantation or adsorption or distillation or combinations thereof. The scrubber may also include treatment with alkali.
Click here to view the patent on Google Patents.