Invented by Prabha N. Ibrahim, Gary Conard Visor, Daiichi Sankyo Inc
Solid forms of compounds modulating kinases refer to the various physical forms that a compound can exist in, such as powders, crystals, or tablets. These solid forms are important because they can affect the bioavailability, stability, and solubility of the compound, which in turn can impact its efficacy and safety.
The market for solid forms of compounds modulating kinases is driven by the increasing prevalence of diseases that are caused by kinase dysregulation. For example, cancer is one of the leading causes of death worldwide, and many cancer therapies target specific kinases that are overexpressed or mutated in cancer cells. As a result, there is a growing demand for solid forms of kinase inhibitors that can effectively target these kinases and improve patient outcomes.
In addition to cancer, there is also a growing interest in using kinase inhibitors to treat other diseases, such as inflammatory disorders and autoimmune diseases. For example, rheumatoid arthritis is a chronic inflammatory disorder that is characterized by the dysregulation of various kinases. By targeting these kinases, researchers hope to develop more effective and targeted therapies for this disease.
The market for solid forms of compounds modulating kinases is also driven by advances in technology and drug discovery. For example, advances in crystal engineering and formulation technology have enabled the development of more stable and bioavailable solid forms of kinase inhibitors. In addition, the use of high-throughput screening and computational modeling has facilitated the discovery of new kinase inhibitors with improved potency and selectivity.
Overall, the market for solid forms of compounds modulating kinases is expected to continue to grow in the coming years, driven by the increasing demand for targeted therapies in the treatment of various diseases. As researchers continue to uncover the roles of kinases in disease pathogenesis, the development of new and more effective kinase inhibitors will be crucial in improving patient outcomes and advancing the field of precision medicine.
The Daiichi Sankyo Inc invention works as follows
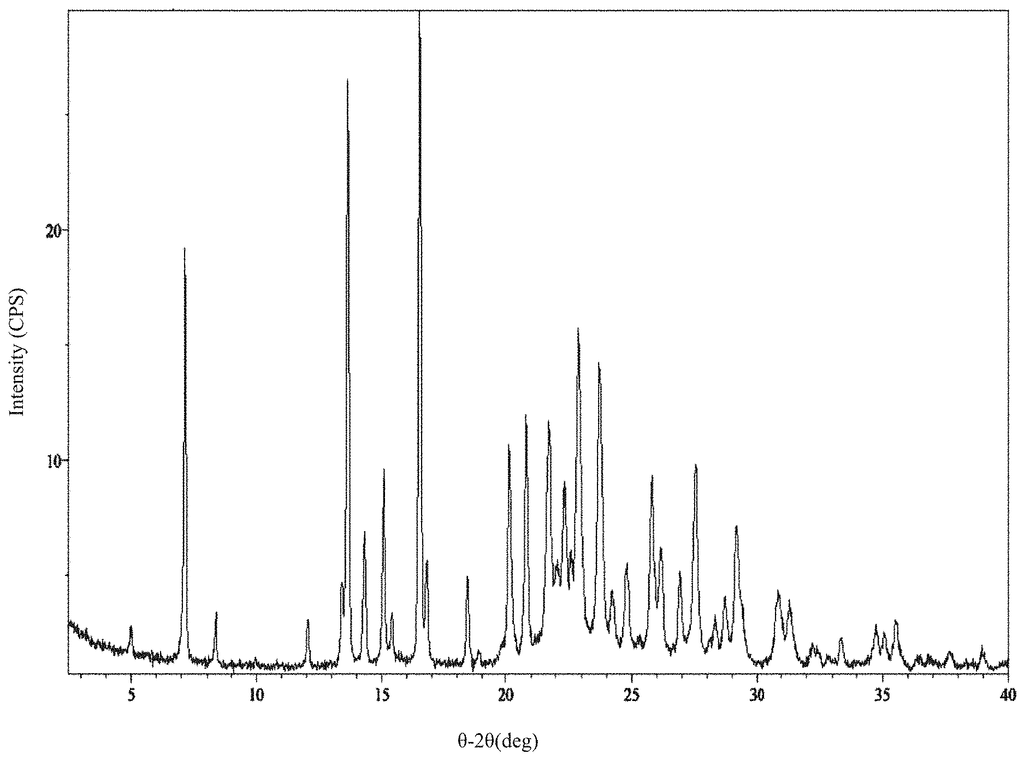
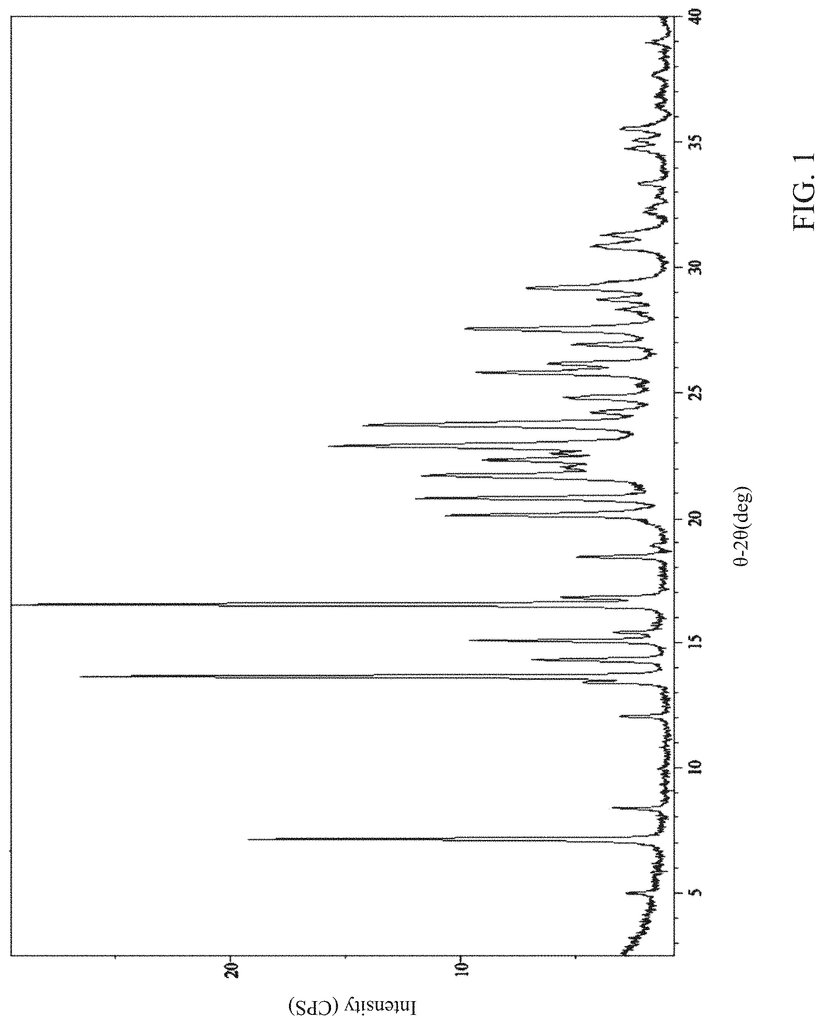
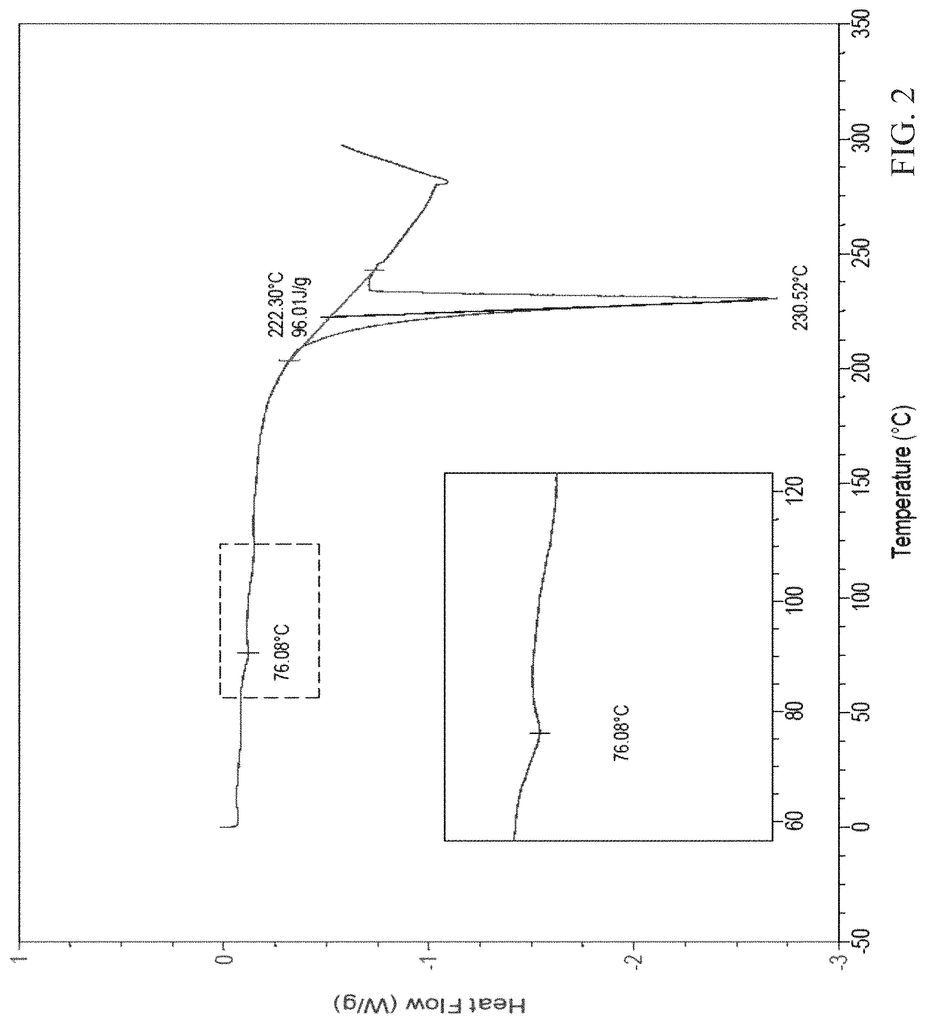
Solid forms of the compound, [5-(5-chloro-1H-pyrrolo[2,3-b]pyridin-3-ylmethyl)-pyridin-2-yl]-(6-trifluoromethyl-pyridin-3-ylmethyl)-amine HCl salt (Compound I) and its free base, active on the receptor protein kinases c-Kit and/or c-Fms and/or Flt3, were prepared and characterized:\nAlso provided are methods of using the solid forms.
Background for Solid forms a compound modulating Kinases
Effective treatments are needed for those who are suffering from or at-risk of a Flt3-mediated disease or condition, such as c-Kit or/or cFms. U.S. Pat. discloses suitable compounds for treating such conditions, including Compound I, and Compound II. No. 7,893,075, U.S. Publication No. Publication No. Publication No. Publication No.
But, Compound I wasn’t known in the specific crystalline forms described herein. Compound II wasn’t also known in the crystalline form described herein.
These needs are met by the present disclosure, which provides solid forms of Compound I and Compound II. The present disclosure also offers crystalline forms either of Compound I and Compound II.
The present disclosure also includes pharmaceutical compositions containing the solid forms of Compound I and Compound II. The disclosure provides methods and processes for making solid forms as well as methods for using them to treat c-Kit, c-Fms, and/or Flt3-mediated diseases and conditions.
Thus, one embodiment refers to a solid version of Compound I. A second embodiment refers to a polymorphic version of Compound 1. Another embodiment is directed at a crystalline version of Compound I. One embodiment of Compound I forms a crystalline form called Compound I Form A. The crystalline form is Compound I Form B in another embodiment. Another embodiment of Compound I forms is Compound I form B. A second embodiment deals with a crystalline version of Compound II.
One embodiment” refers to a pharmaceutical composition that includes a compound chosen from the group comprising Compound I form A, Compound I form B, and Compound I Formula C, as well as crystalline Compound II, and a pharmaceutically acceptable adjuvant.
Another embodiment relates to a method of treating a subject who is suffering from or at-risk of a disease or condition caused by a proteinkinase. This could be c-Fms (or c-Kit), Flt3 (or combinations thereof) and/or macrophages/microglia. It involves administering to the subject a therapeutically-effective amount of Compound 1 Form A, Compound 1 Form B, Compound 2 Form C, Compound 3 or crystalline Compound II
Another embodiment relates to a method of treating a subject with a disease or condition caused by a proteinkinase. This could be c-Fms (or c-Kit), Flt3 (or combinations thereof) and/or macrophages/microglia. It involves administering to the subject a compound containing a therapeutically active amount of Compound 1 Form A, Compound 1 Form B, or Compound 2 Form D, and a pharmaceutically acceptable Excipient
Another embodiment relates to a treatment method for tenosynovial big cell tumor (TGCT) in which Compound I, I, C or Compound I form D are administered to the subject.
Another embodiment relates to a treatment for a subject with pigmented villonodular syndrome (PVNS). It involves administering to the subject a therapeutically-effective amount of Compound I, Compound I, B, or Compound I, C, or Compound I, D, or a combination thereof.
Another embodiment relates to a treatment method for a subject suffering from malignant peripheral nerve sheath tumours (MPNST). It involves administering to the subject a therapeutically-effective amount of Compound I, Compound I, B, or Compound I, C, or Compound I, D, or a combination thereof.
Another embodiment relates to a method of treating a subject with breast cancer or at-risk. It involves administering to the subject a therapeutically active amount of Compound I, Compound I, B, Compound I, C, or Compound I, D, or a combination thereof.
Another embodiment relates to a treatment method for plexiform neurofibromas. It involves administering Compound I, Compound I, B, Compound I, C, and Compound I, D to the subject.
Another embodiment relates to a method of treating melanoma patients, or subjects at risk, with unresectable, metastatic, or metastatic melanoma. It involves administering to the subject a therapeutically-effective amount of Compound I, Compound I, C, and Compound I, D, or a combination thereof.
Another embodiment relates to a method of treating a subject with glioblastoma or at-risk of it. It involves administering to the subject a therapeutically active amount of Compound I, Compound I, B, Compound I, C, or Compound I, D, or a combination thereof.
Click here to view the patent on Google Patents.