Invented by Ming Qi, Thomas Zawodzinski, Shane Foister, University of Tennessee Research Foundation
Rechargeable metal air batteries and regenerative fuel cells require a reversible bifunctional air electrocatalyst to function efficiently. This electrocatalyst is responsible for facilitating the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) in the battery or fuel cell. The ORR and OER are critical processes in the conversion of chemical energy to electrical energy and vice versa.
The market for reversible bifunctional air electrocatalyst is expected to grow significantly in the coming years due to the increasing demand for clean energy sources. The global shift towards renewable energy sources and the need to reduce carbon emissions are driving the demand for rechargeable metal air batteries and regenerative fuel cells.
The use of reversible bifunctional air electrocatalyst in rechargeable metal air batteries and regenerative fuel cells offers several advantages over traditional batteries and fuel cells. These advantages include high energy density, low cost, and environmental friendliness. The use of these batteries and fuel cells can significantly reduce carbon emissions and help in the fight against climate change.
The market for reversible bifunctional air electrocatalyst is highly competitive, with several players offering a wide range of products. The major players in the market include BASF SE, Johnson Matthey, Umicore, and Clariant AG. These companies are investing heavily in research and development to improve the efficiency and performance of their products.
The market for reversible bifunctional air electrocatalyst is expected to grow at a CAGR of 10.5% during the forecast period 2021-2026. The Asia-Pacific region is expected to dominate the market due to the increasing demand for clean energy sources in countries such as China and India.
In conclusion, the market for reversible bifunctional air electrocatalyst for rechargeable metal air battery and regenerative fuel cell is rapidly growing due to the increasing demand for clean energy sources. The use of these batteries and fuel cells can significantly reduce carbon emissions and help in the fight against climate change. The major players in the market are investing heavily in research and development to improve the efficiency and performance of their products. The market is expected to grow at a CAGR of 10.5% during the forecast period 2021-2026, with the Asia-Pacific region dominating the market.
The University of Tennessee Research Foundation invention works as follows
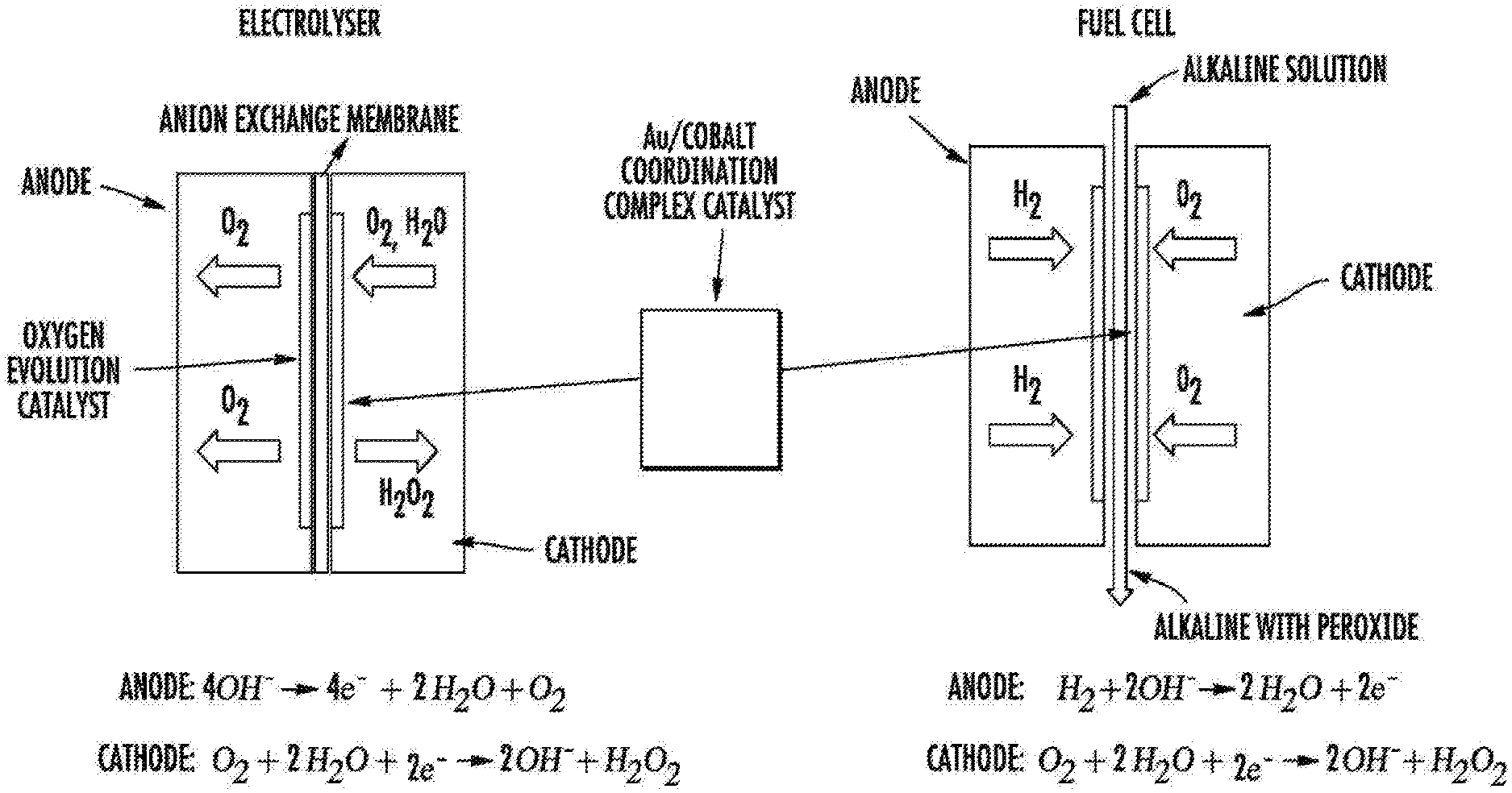
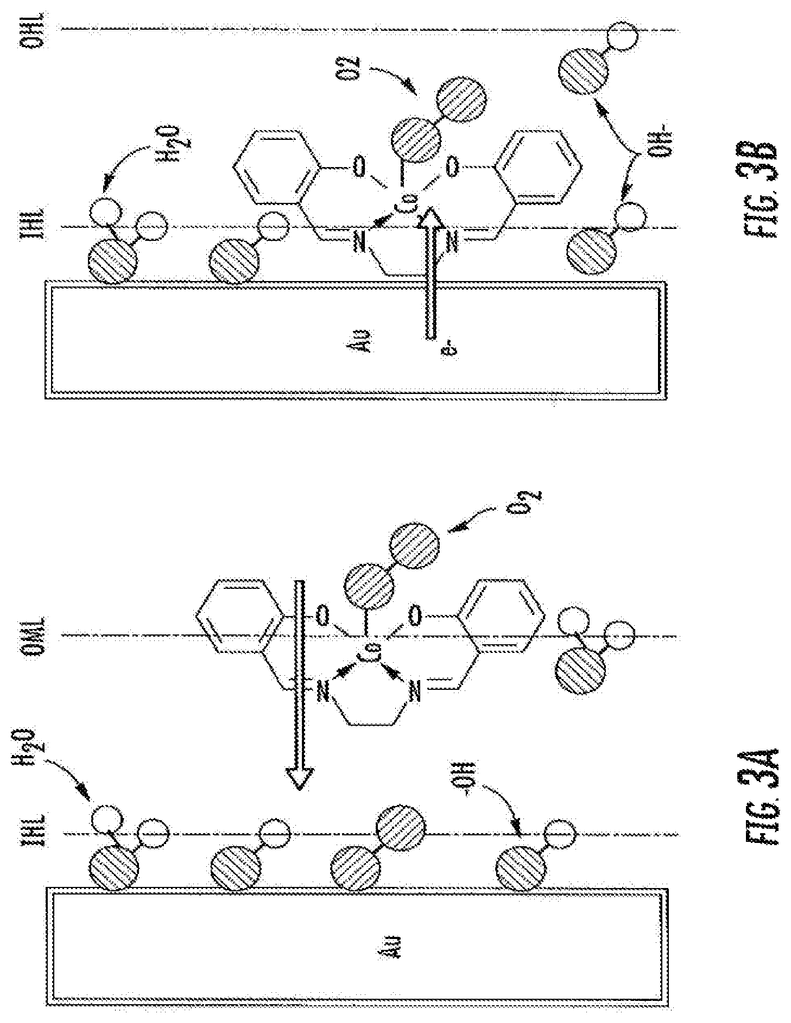
An electrochemical device includes an air-electrode in communication with an aqueous hydrogen peroxide solution in a storage tank, a Lithium electrode, either a gas diffusion or air electrode layer, and a separater layer in direct contact with both the catalyst and the catalyst layer. The catalyst layer contains a catalyst that is used for reversible two-electron oxygen reduction. The catalyst is made of gold and polymers or cobalt complexes. The cobalt complex is a cobalt-chelated ion, chelated with a tetradentate chelating organic ligand.
Background for Reversible Bifunctional Air Electrocatalyst for Rechargeable Metal Air Battery and Regenerative Fuel Cell
The development of alternative and renewable sources is important due to the environmental concerns that are associated with fossil fuels as well as energy security. There are many types of renewable sources, such as wind power, solar energy, biomass fuels and geothermal power. Some of these sources, like wind and solar energy, aren’t constant or easily transportable. It is therefore often convenient or necessary to convert the energy source to electrical energy to transport it and then store it in a variety of ways, such as as heat using thermal storage, or chemical energy stored in batteries or capacitors.
Also, the automotive industry is making great efforts to replace combustion engines with cleaner technology in order to reduce pollution. Fuel cells and batteries are two types of electrochemical devices that could be used as replacements. Fuel cells convert chemical energy (from an environmentally friendly fuel, such as hydrogen), into electricity by electrochemically reacting with oxygen. For this approach to work, it would be necessary to develop and/or build new fuel production methods, fuel storage systems, and national transport networks.
Batteries and super capacitors store and transfer electrical energy in the form of chemical energy, and electrical field energy. Batteries, in particular, store electrical energy through chemical oxidation and reduction energy. They then convert this chemical energy into electrical energy during use. Chemical energy can also be stored within the active materials in a negative battery electrode. During discharge, electrons are sent from the negative electrode to the positive through an external circuit. This powers the load and completes the discharge reaction. At the same time, ions travel directly from the negatively charged side to the positively charged side via an electrolyte. In secondary batteries, the electrons and the ions move in the opposite directions during the recharge. The use of rechargeable batteries in automobiles could be a viable option to replace combustion engines, given the existing electrical grid system.
The high theoretical energy density of lithium-air batteries (i.e. 12 kWh/kg) is a subject of intense scientific research. Lithium metal anodes emit lithium ions when they discharge. The electron travels through an external circuit, while lithium ions are transported through the electrolyte to the cathode. The oxygen combines with electrons and lithium to complete the reaction, resulting in lithium oxide (i.e. Li2O or Li2O2). When charging, the lithium oxide or peroxide is decomposed to produce oxygen. The lithium ions then travel to the anode to be reduced to lithium metal. The oxygen electrode may be porous to store solid products produced by the reaction between Li ions and O2 (i.e. Li2O and Li2O2) in the discharge cycle and usually include a catalyst to promote reactions. Four different types of Li-Air batteries have been proposed, depending on the electrolyte. These are aprotic (liquid), aqueous (water), solid state and mixed aqueous/aprotic. The overpotential of aprotic Li-Air Batteries to return to oxygen and lithium is high, typically over 1.5V.
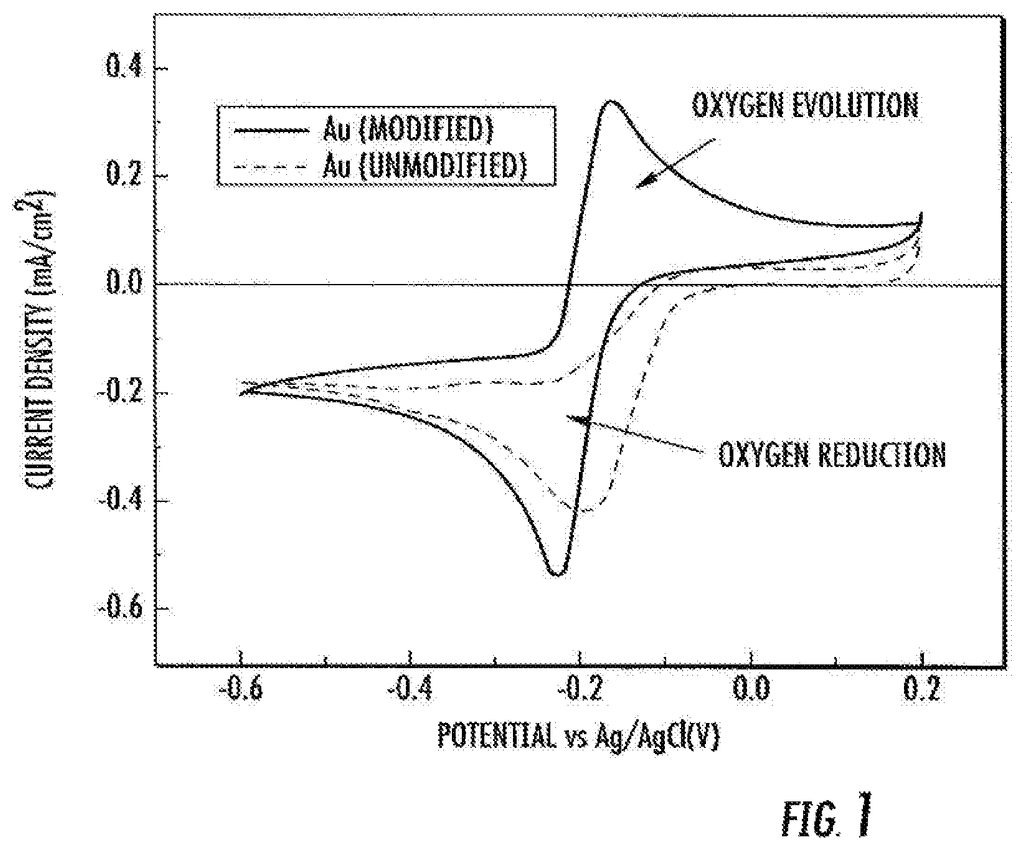
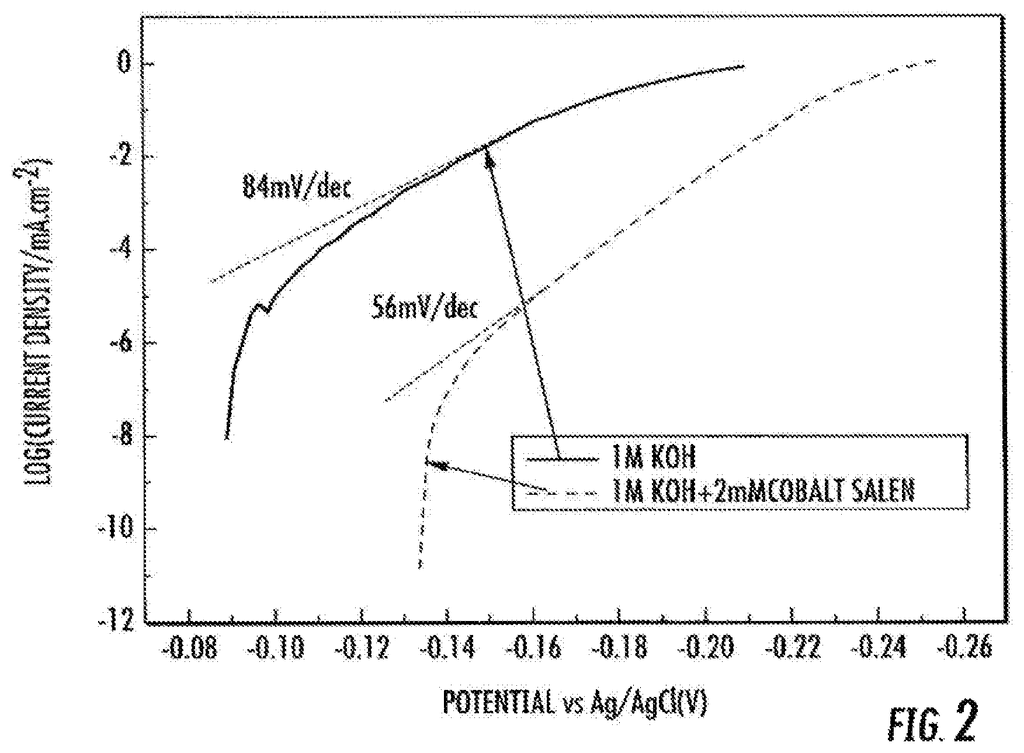
There is a need to develop improved electrochemical systems such as metal-air batteries and robust bifunctional catalysers (i.e. catalysts that reduce both the discharge and charge overpotentials) for such systems. This can improve efficiency and recharge rate, for example. There is a requirement for reversible catalysers that can both promote the oxygen reduction (ORR), and the oxygen evolution (OER) reactions.
Hydrogen Peroxide is a commodity chemical of great importance.” Hydrogen peroxide is used in bleaching agents (e.g. the paper industry), as well as cleaning and disinfection agents. Hydrogen peroxide is also used in the cosmetics industry and as propellant. The majority of hydrogen peroxide produced today is made via the anthraquinone-oxidation process. This results in a large amount of chemical waste. Since the anthraquinone-oxidation process can be difficult to perform on a small level, hydrogen peroxide production generally involves transport and storage. This can cause safety concerns. Hydrogen peroxide is also produced at a smaller-scale via electrochemically or by direct synthesis of hydrogen and oxygen. The hydrogen peroxide can be produced locally, reducing the need for transport and storage. Direct synthesis can have its own risks (e.g. due to the flammability in mixtures of oxygen and hydrogen gases). Recently, efforts have been made in order to improve ORR catalysts for the electrochemical production of hydrogen peroxide. However, there is still need for higher-performance ORR catalysts.
According to some embodiments, the subject matter disclosed herein provides a catalyst that is capable of reversible two-electron oxygen reduction. In certain embodiments, the catalyst comprises (a) gold and (b), a polymer or cobalt complex coordination complex. In one embodiment, the cobalt complex is a cobalt-chelated ion. In some embodiments, the tetradentate organic chelating ligand is N,N?-bis(salicylidene)ethylenediamine or a derivative thereof.
In some embodiments, a cobalt solution in contact with gold contains the complex. In certain embodiments, a cobalt coating is used to cover a surface of an gold structure. In some embodiments the gold is contained in a small particle. In some embodiments the particle can be a nanoparticle. The nanoparticle may have a diameter between about 1 nm and about 20 nm.
In some embodiments the cobalt complex coordination has a formula (I) structure:
In some embodiments, one or more of R1,R2, R3, R4, and R5, R6, R7, R8, and R9 are conducting moiety selected from the group consisting thiophenyl and pyrrolyl;?NHC6H6,?SC6H5;?CHCHC6H5;??C?CH, and a conductivity polymer. In certain embodiments, R1, R2, and R3, are conducting moieties selected from the group consisting pyrrolyls,?NHC6H6, and?SC6H5s;?CH??CH?C6H5?C6H5?C?CH; and conducting polymers. In some embodiments one or more R2, R3, C6, R7, and R8 are thiophenyl. Optionally, both R3 andR7 can be thiophenyl. In some embodiments R can be phenylene, or ethylene substituted.
In some embodiments, the catalyst comprises a polymer of the cobalt coordination complex, wherein the polymer is formed by bonds between the organic chelating ligands of individual monomer units of the cobalt coordination complex, optionally wherein the polymer is prepared by electropolymerization. The polymer can be present in some embodiments as a coating on the surface of gold-containing structures.
According to some embodiments, the subject matter disclosed herein provides an air electrode that contains a catalyst. According to some embodiments, the subject matter disclosed is an air electrode comprising the catalyst disclosed herein.
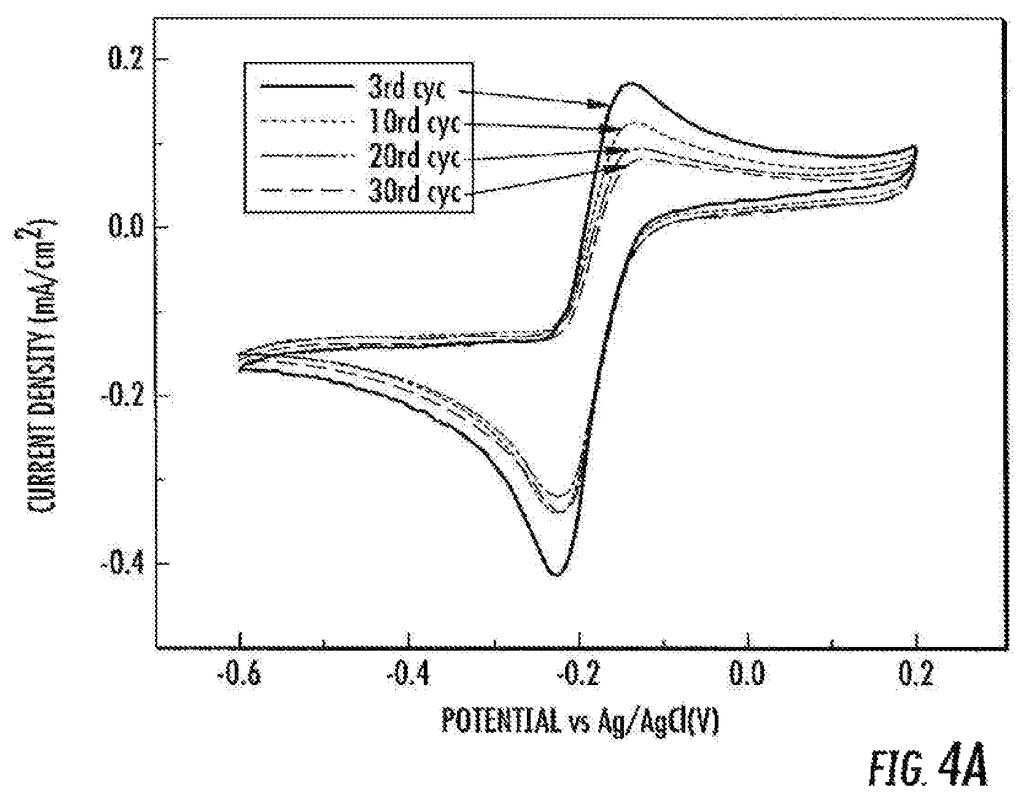
According to some embodiments, the subject matter disclosed herein provides a method of performing a two-electron reduction of oxygen. In certain embodiments, the method includes contacting oxygen with a catalyst, as disclosed herein. Optionally, where the reduction is reversible, or near-reversible, this can be done.
According to some embodiments, the subject matter disclosed herein provides a method of producing hydrogen peroxide. In certain embodiments, the method includes contacting oxygen in an aqueous mixture with a catalyst disclosed herein. This produces hydrogen peroxide.
In accordance with certain embodiments, a method for preparing a catalyst for oxygen reduction by two-election is provided. In some embodiments, the method comprises: (a) providing a solution comprising a cobalt coordination complex, optionally wherein the cobalt coordination complex comprises a cobalt ion chelated by a tetradentate organic chelating ligand, optionally wherein the tetradentate organic chelating ligand is N,N?-bis(salicylidene)ethylenediamine or a derivative thereof; (b) providing a structure comprising a gold surface; (c) contacting the structure with the solution from step (a); (d) electropolymerizing the cobalt coordination complex to form an electropolymerized film covering at least a portion of the gold surface.
The present disclosed subject matter aims to provide catalysts that reduce oxygen by two electrons (e.g. reversible reduction of oxygen), electrochemical cells containing the catalysts, as well as methods for producing the catalysts and ways of using them to reduce oxygen or provide hydrogen peroxide.
As the description continues, it will be clear that other objects are being achieved by the subject matter as described below.
The subject matter disclosed herein will be described in greater detail. However, the presently disclosed matter can be embodied differently and should not necessarily be limited to the embodiments described below and in the accompanying examples. These embodiments have been provided to make this disclosure complete and accurate, and to fully convey the scope and nature of the embodiments for those in the know.
All references, including but without limitation, all patents, applications for patents and publications thereof and scientific Journal articles are incorporated by reference herein in their entirety to the extent they explain, provide background information, or teach methodologies, techniques and/or compositions used herein.
I. Definitions
The following terms should be understood by anyone with a basic understanding of the subject matter. However, these definitions will help to clarify the disclosure.
Click here to view the patent on Google Patents.