Invented by Scott J. Hollister, Glenn E. Green, University of Michigan
The device is made up of two bellows that are connected by a porous membrane. The bellows are designed to expand and contract in response to changes in pressure, which helps to maintain a stable airway. The porous membrane allows for the growth of new tissue, which helps to support the trachea and prevent further narrowing.
One of the key advantages of the porous bellowed tracheal reconstruction device with bidirectional bellows is that it can be customized to fit the patient’s specific needs. The device can be adjusted to accommodate different levels of tracheal stenosis, and it can also be tailored to fit the patient’s anatomy. This makes it a highly effective solution for patients who have complex tracheal problems.
Another advantage of this device is that it is minimally invasive. Unlike traditional tracheal reconstruction surgeries, which require large incisions and lengthy recovery times, the porous bellowed tracheal reconstruction device with bidirectional bellows can be inserted through a small incision. This reduces the risk of complications and allows for a faster recovery time.
The market for porous bellowed tracheal reconstruction devices with bidirectional bellows is expected to continue to grow in the coming years. As more patients are diagnosed with tracheal stenosis, there will be an increasing demand for effective and minimally invasive treatments. The porous bellowed tracheal reconstruction device with bidirectional bellows is well-positioned to meet this demand, and it is likely that we will see more of these devices being used in hospitals and clinics around the world.
In conclusion, the market for porous bellowed tracheal reconstruction devices with bidirectional bellows is a promising one. These devices offer a highly effective and minimally invasive solution for patients with tracheal stenosis, and they are likely to become more widely used in the coming years. As the medical community continues to explore new and innovative treatments for tracheal problems, the porous bellowed tracheal reconstruction device with bidirectional bellows is sure to play an important role in improving the lives of patients.
The University of Michigan invention works as follows
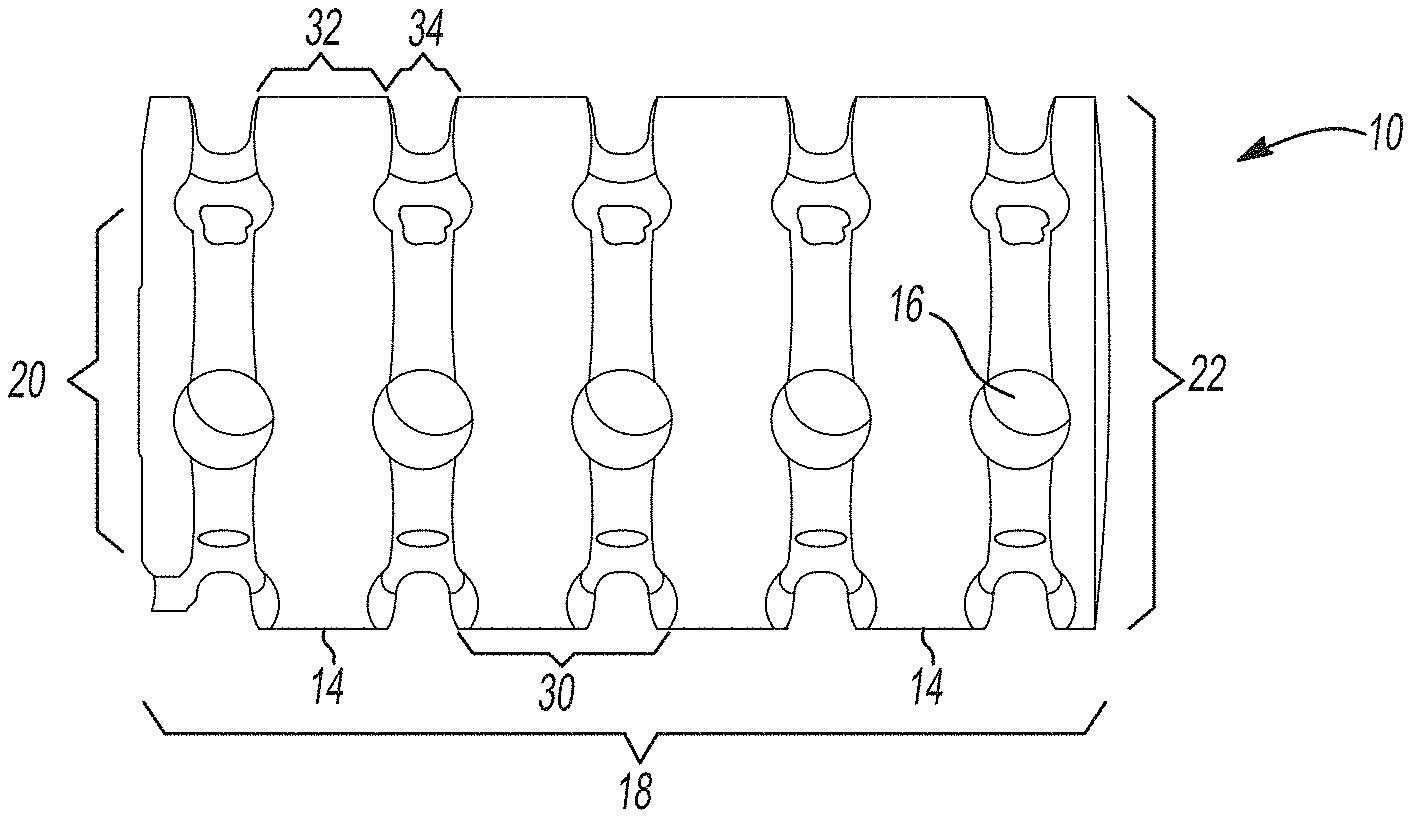
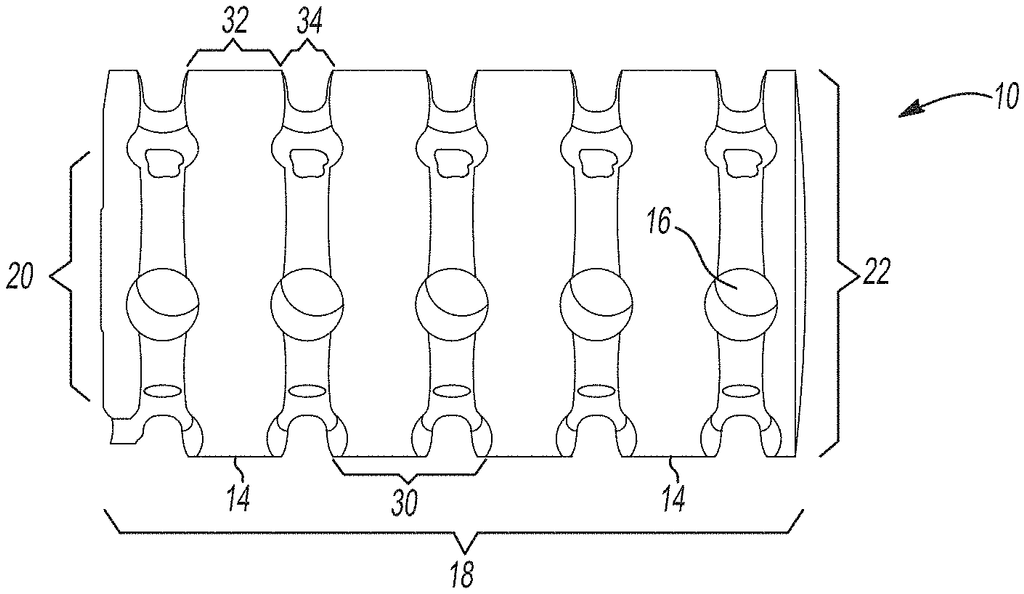
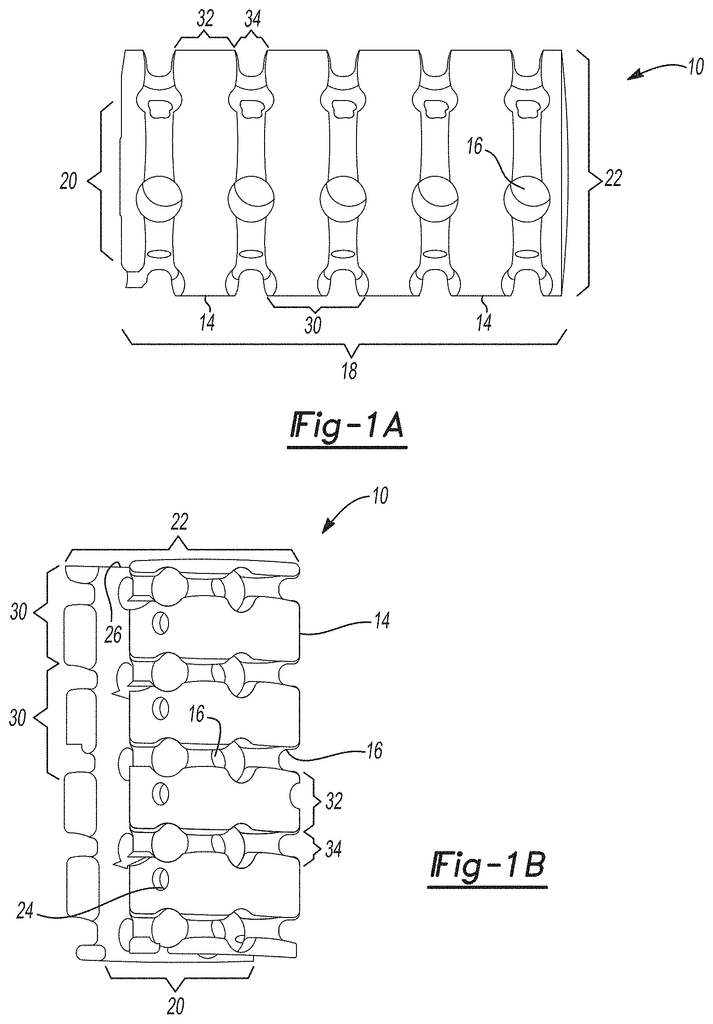
Implantable splinting devices that support a passageway defect in patients. Made from one or more support structure, such as a polymer or a combination of a cellulized tissue matrix and polymer, the structural component is designed to conform to the passageway. There are also a number of pores in the structural component. The implantable surgical device can be placed around the trachea, the bronchi, and an esophagus of a patient. An implantable splinting system can also be used to place between the trachea and the esophagus.
Background for Porous bellowed tracheal reconstruction device with bidirectional bellows
The present technology is about materials and implants that are useful in maintaining patency in passageways, such as airways, or treating conditions where passageways in humans or animals have collapsed or become weaker, like in tracheomalacia and tracheoesophageal fstula. Tracheomalacia or tracheobronchomalacia (TBM) are congenital or acquired deficiencies of tracheal and/or bronchial cartilages. Flaccidity in the supporting tracheal and bronchial cartilages can lead to airway obstruction, respiratory difficulties, and even death. TBM is a condition that affects approximately 1 in 2100 children. It can cause respiratory problems, chronic cough, wheezing and recurrent infections. In severe cases, it can lead to life-threatening complications. TBM can be fatal in infants and adults who are unable to respond to medical treatment or suffer from life-threatening symptoms. The severe cases may lead to death, or require tracheostomy and ventilation for 2-3 years. This is a significant burden on the child’s family. The current treatment options include surgery, mechanical ventilation and implantation of tracheal tubes. As noted in the Cochrane reviews and other studies, all surgical/mechanical interventions have had a high rate of failure, morbidity, and mortality.
TBM surgery/mechanical devices can be divided into stents (devices placed within or near the passageway, e.g. the trachea) and splints (devices externally placed around the passageway, e.g. the trachea). Although stents are a simpler surgical option, they can cause complications such as stent migration and granulation tissue that may lead to secondary obstruction. This could also make it necessary for multiple procedures or even death. Additionally, metal and conventional silicone tracheal devices have caused problems in the placement, stent distortion and migration, tissue growth and stability, and reduced adjustability. These complications have led the U.S. Food and Drug Administration to warn against the use tracheal-stents.
However, splints can also be prone to tracheal erosion or granuloma formation. The stiffness of the splints is thought to be the reason for these adverse effects. There are concerns that splints made from too stiff materials can restrict tracheobronchial development, leading to more complications in the long-term, particularly for children. In rats, tracheal banding was found to not only restrict tracheal development but also to reduce overall lung volume, alveoli, and overall body growth. Devices that aren’t stiff enough can cause or maintain tracheobronchial patency, and they also easily move from their intended position. Although degradable devices may be a partial solution, it has been shown that they degrade too quickly, leaving the trachea with insufficient time to remodel and become structurally competent. This can lead to TBM or other passageway problems. Implantable devices that address these issues would be beneficial to reduce mortality, improve patient outcomes and improve growth in order to allow the passageway with the defect to become structurally competent. This would also improve the animal’s quality of life.
This section gives a summary of the disclosure, but is not an exhaustive disclosure of its full scope and all its features.
In various embodiments, this technology provides an implantable device that supports a passageway defect in a patient or animal. It is made up of a polymer. One or more support structures may be used to create a structural component and multiple pores. The implantable device can be placed around at most a portion of the passageway of a patient from the following group: a trachea or bronchi, an stomach, an esophagus, and a blood vessel. In certain variants, the implantable splinting system may be used to place the device between the patient’s trachea or esophagus.
In another variation, an implantable surgical device to support a defect in the passageway of a person or animal subject consists of one or more support elements together defining a structural element that substantially conforms with at least a part of the passageway. One or more support structures are made of a biocompatible, biomedically-acceptable polymeric material. Optionally, one or more of the support structures may include a longitudinal opening within them to allow for the placement of the implantable device over the passageway. An implantable splinting tool has a number of apertures that are defined in at most a portion the support structures. These apertures can receive a suture to attach the implantable device to at minimum a portion the passageway.
The present technology, in other aspects, provides an implantable device to support a defect in the passageway of a person or animal subject. It comprises one or more support structure that define a body or structural component that conforms substantially to at least a part of the passageway. An implantable device includes a plurality apertures that are defined in at most a portion the one or more support structure. One or more support structures are made of a biocompatible, biomedically acceptable, polymeric material. The body or structural components can resist displacement up to 10% under a compression load of approximately 50N. However, the body or structural part is capable of allowing outward displacement of at least 100 percent of its initial diameter under stress conditions of around 50N. This implantable splinting device can provide sufficient structural support for the passageway to resist compression forces and torsion in vivo. It also allows flexibility to allow growth and revascularization.
In other aspects, the present technology offers methods for making an implantable surgical device that can be used to treat a defect in the passageway of a person or animal. This involves forming a biocompatible, biomedically acceptable, or biomedically acceptable, polymeric material into an U-shaped structural part comprising a plurality o apertures and a plurality o bellows. The U-shaped structural element substantially conforms to at most a portion selected from the group consisting, among others, bronchi, esophagus, esophagus, a, a bronchi and a esophagus, a blood vessel, esophagus, trachea bronchi, esophagus, a trachea bronchi, esophagus, esophagus, a trachea bronchi, a esophagus, a a a a a a a a a a a a a a a sophagus, a a a a a a a a a a a a and a a a a a a a a a a a a a a ensophagus, a a a a a a a a a a a a The implantable splinting device can be designed using specific medical images that are specific to each patient. The implantable splinting devices can be customized to fit the needs of a specific animal or human.
In another variation, a method for making an implantable device to treat a passageway defect in a patient of any animal or human is described. This method involves designing the structural component of the implantable device using a set medical image data that identifies the passageway defect. Next, the structural component is laser sintered with a biodegradable plastic. Finally, the structure is equipped with a number of pores that can be used to attach the implantable splinting devices to at least some of the passageways.
In another aspect, the present technology includes an implantable splinting system for supporting a trachealesophageal fistula (in a human or animal patient) comprising a polymer. An implantable splinting device could include a U-shaped structural component for the tracheal and an U-shaped structural part for the esophageal. These structural components can be made from multiple support structures and may include a number of apertures. The implantable splinting device could also include a first and second component. The hinge joins the first and second components in certain variants.
The present technology also provides a method to make an implantable splinting tool for treating passageway defects in humans or animals. It involves: providing a polymer; forming the polymer into U-shaped structural components by forming a series support structures and integrating multiple pores within the support structures.
The unique implantable splinting technology of the current technology has the advantage of being able to design from standard dimensions and lengths of the blood vessels, trachea, and bronchi or from patient-specific medical image data before surgery.
Further areas will be apparent from the description herein. This summary and the specific examples are for illustration purposes only. They do not limit the scope of disclosure.
DRAWINGS
The drawings herein are intended to illustrate selected embodiments only and not all possible implementations. They do not limit the scope and meaning of the present disclosure.
FIGS. “FIGS.
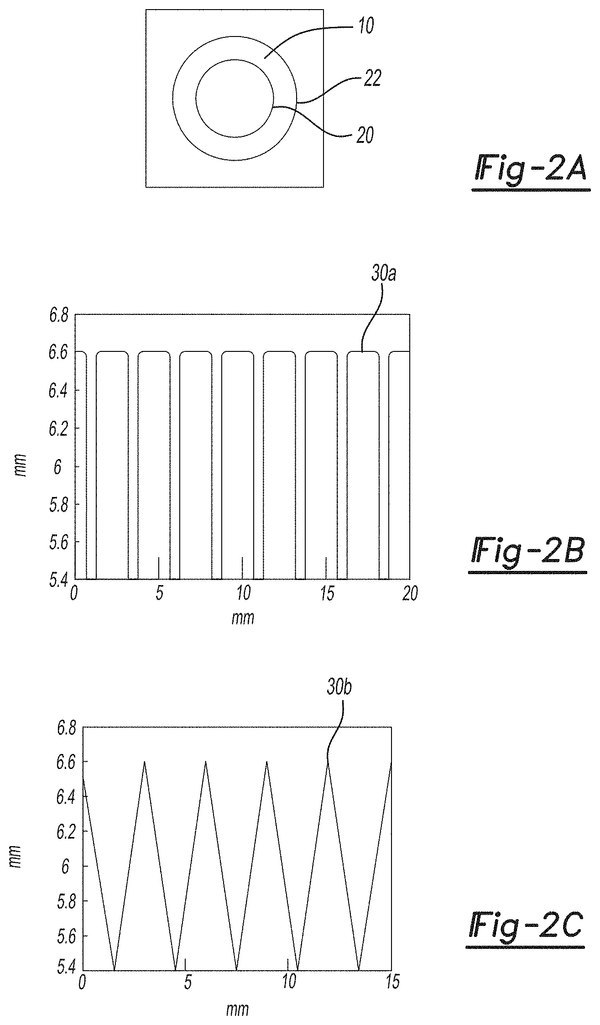
FIGS. 2A-2C are in-plane lumens (2A), square extrusions of bellow waves (2B) and triangle extrusions of bellow waves (2C) according certain aspects of the current teachings.
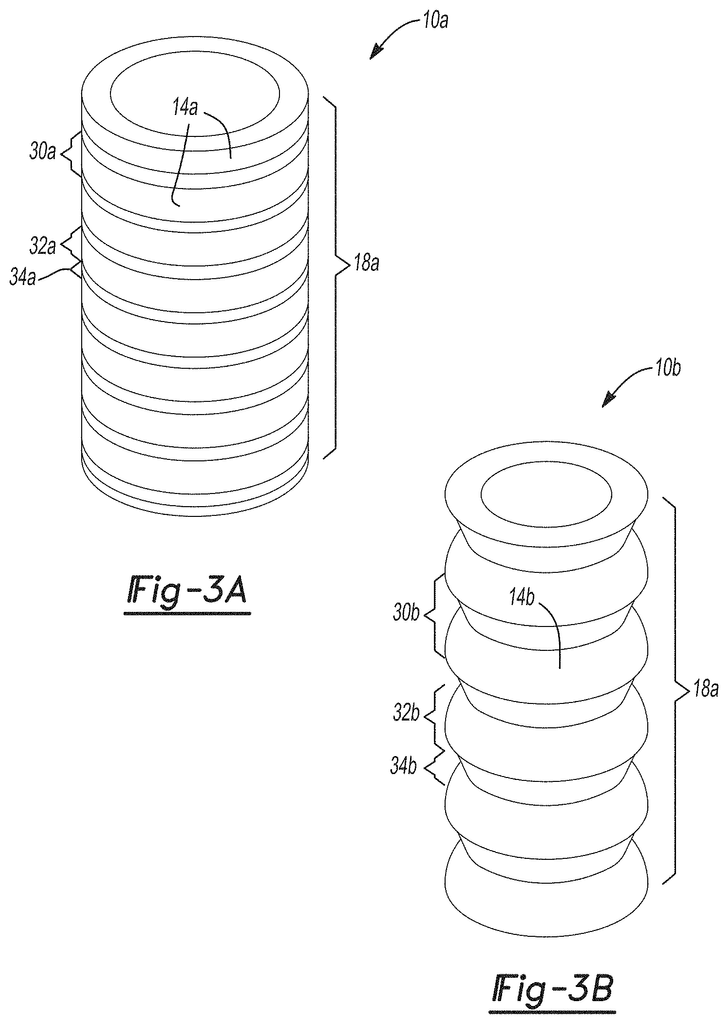
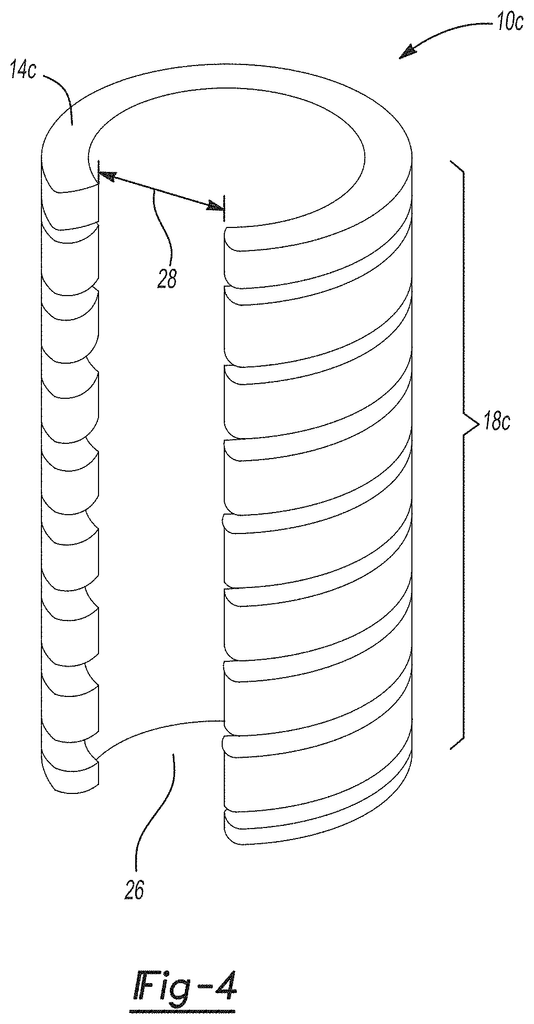
FIGS. 3A-3B are a square and triangle wave bellows (3A-3B) respectively of an implantable surgical device, according to certain aspects.
FIG. “FIG.
FIGS. “FIGS.5” 5A-5B depict an implantable splinting tool with a square and wedge bellow and a plurality openings (5A), and one portion of the plurality apertures configured for a suture needle (5B) according certain aspects of this teachings.
FIGS. “FIGS.
Click here to view the patent on Google Patents.