Invented by Andrea MAHR, Toni Weinschenk, Oliver Schoor, Jens FRITSCHE, Harpreet Singh, Lea STEVERMANN, Immatics Biotechnologies GmbH
Peptides are short chains of amino acids that play a crucial role in many biological processes. They have been studied extensively for their potential as therapeutic agents for cancer. Peptide-based drugs have several advantages over traditional chemotherapy drugs, including greater specificity and fewer side effects.
Peptide combinations, or peptide cocktails, are a combination of two or more peptides that work together to target cancer cells. These combinations have shown promising results in preclinical studies and are currently being evaluated in clinical trials.
Immunotherapy, on the other hand, is a type of cancer treatment that harnesses the power of the immune system to fight cancer. Immunotherapy drugs work by stimulating the immune system to recognize and attack cancer cells. This approach has shown remarkable success in treating certain types of cancer, including melanoma and lung cancer.
The market for peptides, peptide combinations, and immunotherapy for various tumors is expected to grow significantly in the coming years. According to a report by Grand View Research, the global peptide therapeutics market is expected to reach $48.4 billion by 2025, with cancer being one of the major therapeutic areas.
Several pharmaceutical companies are currently developing peptide-based drugs for cancer treatment. For example, AstraZeneca is developing a peptide-based drug called MEDI9197 for the treatment of solid tumors. Another company, OncoPeptides, is developing a peptide-based drug called Ygalo for the treatment of multiple myeloma.
In addition to peptide-based drugs, several immunotherapy drugs are also being developed for cancer treatment. Keytruda, developed by Merck, is an immunotherapy drug that has been approved for the treatment of several types of cancer, including melanoma, lung cancer, and head and neck cancer. Another immunotherapy drug, Opdivo, developed by Bristol-Myers Squibb, has been approved for the treatment of several types of cancer, including melanoma, lung cancer, and kidney cancer.
In conclusion, the market for peptides, peptide combinations, and immunotherapy for various tumors is rapidly growing. These therapies have shown promising results in preclinical and clinical studies and are expected to play a significant role in cancer treatment in the coming years. With an increasing number of pharmaceutical companies investing in research and development in this field, we can expect to see many more innovative therapies being developed in the near future.
The Immatics Biotechnologies GmbH invention works as follows
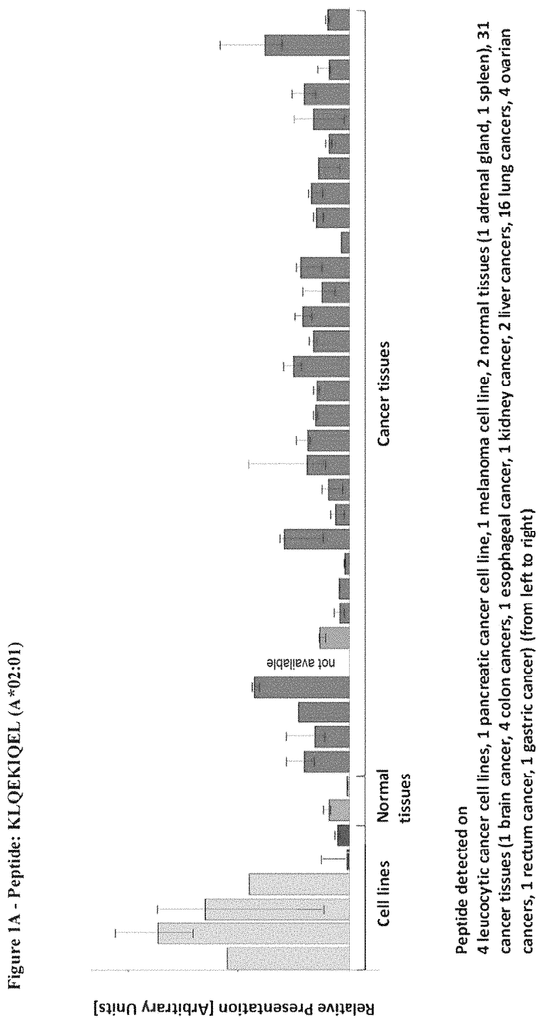
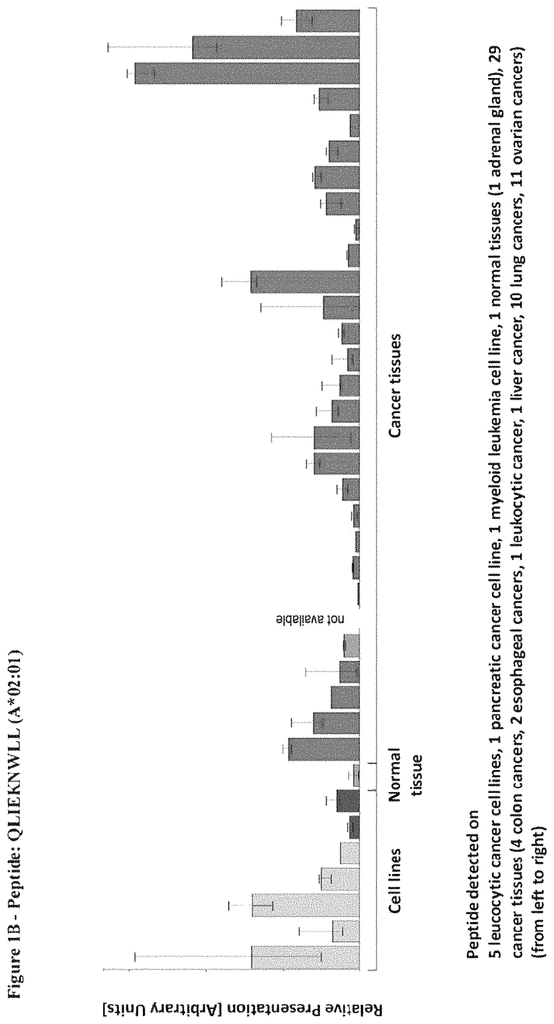
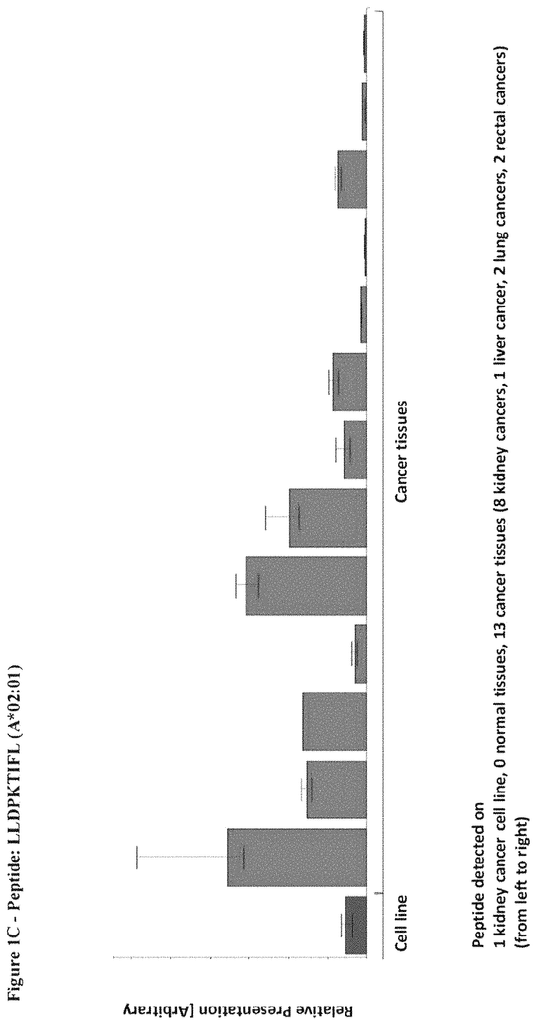
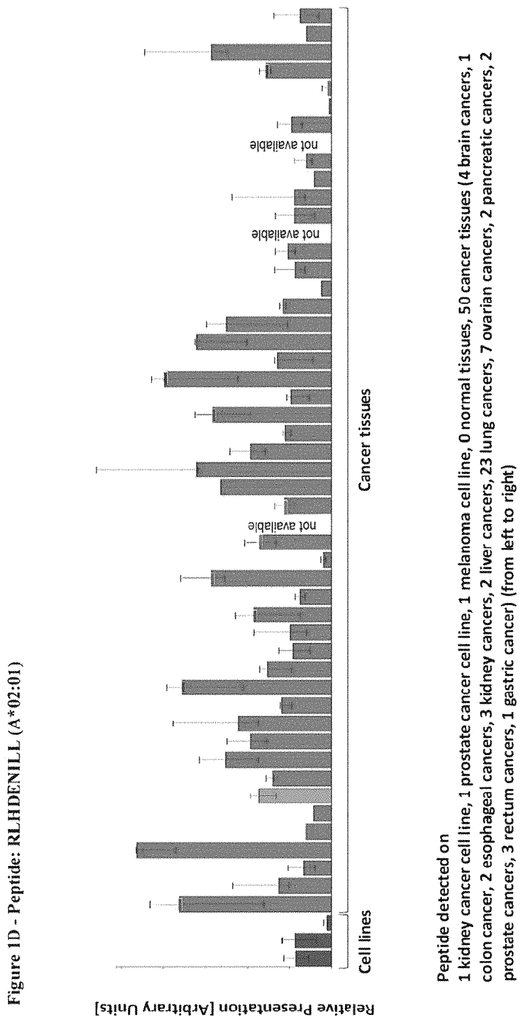
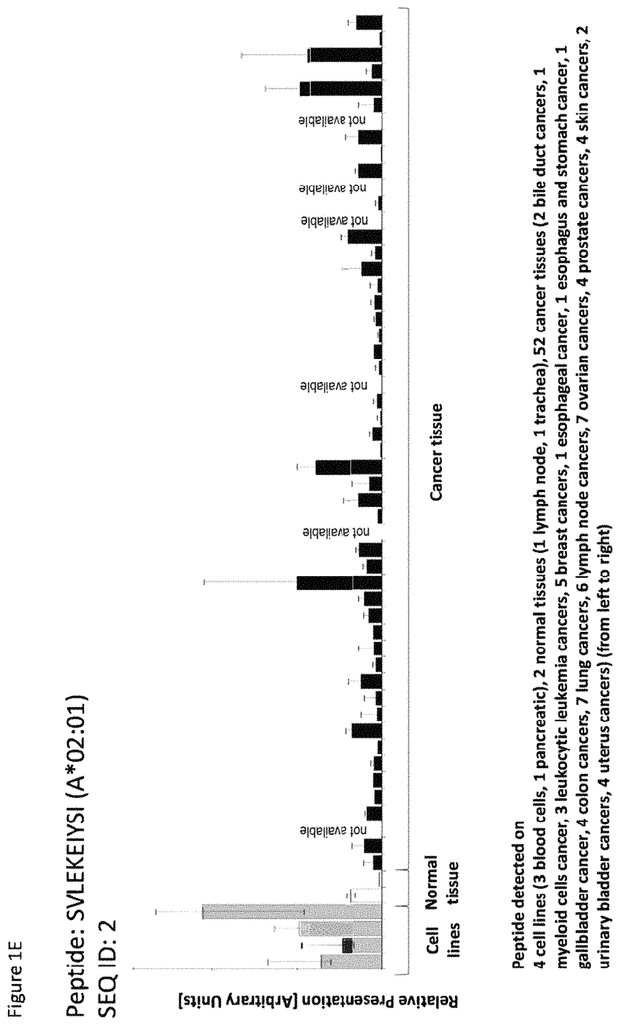
The invention concerns peptides and proteins as well as cells that can be used in immunotherapeutic procedures. The present invention is particularly relevant to immunotherapy for cancer. The invention also relates to tumor-associated T cell peptide epitopes. These can be used alone or in combination, to stimulate immune responses or T cells ex-vivo for transplantation into patients. Antibodies, soluble T cell receptors and other binding molecules can target peptides bound to the major histocompatibility complicated (MHC) or peptides in general.
Background for Peptides, peptide combinations and immunotherapy for various tumors: a review
According to the World Health Organization, cancer was one of the top four non-communicable diseases in the world in 2012. For the same year, colorectal cancer, breast cancer and respiratory tract cancers were listed within the top 10 causes of death in high income countries (www.who.int/mediacentre/factsheets/fs310/en/).
Epidemiology
In 2012, worldwide, it was estimated that 14.1 million cancer cases were diagnosed, 32.6% of cancer patients (within five years) had cancer, and 8.2% of cancer deaths occurred (Ferlay, Bray, et. al. 2013).
The current invention focuses specifically on glioblastoma, chronic lymphocytic (CLL), acute myeloid (AML), and non-small and small cell lung carcinoma (NSCLC) respectively.
The United States has the highest incidence of GB, with an age-adjusted rate of 3,19 per 100,000 residents. GB is a disease with a poor prognosis. It has a survival rate for the first year of only 35%, and a survival rate for five years that is less than 5%. “The risk factors of GB are male gender, older age, and ethnicity (Thakkar et. al.,2014).
CLL is the most common form of leukemia in Western countries, where it accounts for about one-third of all leukemias. The incidence rates in the US are comparable to those in Europe. New cases are estimated at 16,000 each year. CLL is commoner in Caucasians compared to Africans. It’s rarer among Hispanics, Native Americans, and Asians. CLL rates in people of Asian descent are three times lower than those in Caucasians. (Gunawardana, et. al., 2008). The five-year overall survival for patients with CLL is about 79% (www.cancernet/cancer-types/leukemia-chronic-lymphocytic-cll/statistics).
Lung cancer is the second most common cancer type in the world and the number one cause of cancer-related death in many countries. Lung cancer can be divided into non-small and small cell lung carcinoma. NSCLC is comprised of the histological types large cell carcinoma, squamous-cell carcinoma and adenocarcinoma. It accounts for 85% all lung cancers diagnosed in the United States. The incidence of NSCLC, which includes current and former smokers, is closely related to smoking prevalence.
Therapy
Breast Cancer
The standard treatment for patients with breast cancer depends on several factors: the tumor stage, hormone receptor status, and HER2 expression. Standard care involves complete surgical removal of the tumor, followed by radiation treatment. The chemotherapy, mainly with anthracyclines or taxanes, can be initiated before or after the resection. Tratuzumab is given to patients with HER2-positive tumours in addition the chemotherapeutics. (S3-Leitlinie Mammakarzinom 2012). Breast cancer is an immunegenic cancer entity, and different types infiltrating immuno cells in primary tumors have distinct prognostic or predictive significance. Many early phase immunotherapy studies have been performed in breast cancer patients. “Emens (2012)” reports that clinical data are now emerging on the effects of immune-checkpoint modulation using ipilimumab or other T cell activating antibodies for breast cancer patients.
Chronic Leukemia
While CLL is currently incurable, some patients only show a slow progression or worsening symptoms. There are several treatment options available for patients who have symptoms or a rapidly progressing condition. They include chemotherapy, targeted therapies, immune-based treatments like monoclonal antibody, chimeric Antigen-Receptors (CARs), active immunotherapy and stem cell transplants.
Several ongoing and completed trials are based upon engineered autologous CAR-modified T cell with CD19 specificity” (Maus et. al.,2014). Only a minority of patients have shown detectable CARs or persistent CARs. Porter et al. have detected two complete responses and one partial response in CAR T cell trials. and Kalos et al. (Kalos et al., 2011; Porter et al., 2011).
Active Immunotherapy includes strategies such as gene therapy, whole modified tumour cell vaccines (WMCV), DC-based vaccines, and tumor-associated antigen-derived peptides.
Several TAAs over-expressed by CLL are suitable for vaccines. These include fibromodulin (Mayr et al., 2005), RHAMM/CD168 (Giannopoulos et al., 2006), MDM2 (Mayr et al., 2006), hTERT (Counter et al., 1995), the oncofetal antigen-immature laminin receptor protein (OFAiLRP) (Siegel et al., 2003), adipophilin (Schmidt et al., 2004), survivin (Granziero et al., 2001), KW1 to KW14 (Krackhardt et al., 2002) and the tumor-derived IgVHCDR3 region (Harig et al., 2001; Carballido et al., 2012). The RHAMM-derived R3 was used as a vaccine in a phase I clinical study. 5 of 6 patients had detectable R3-specific CD8+ T-cell responses (Giannopoulos et al., 2010).
Colorectal Cancer
Depending on the stage of colorectal (CRC), different standard treatments are available for colon cancer and rectal carcinoma. The standard procedures for CRC include surgery, radiotherapy, chemotherapy, and targeted therapy (Berman, et. al.,2015a; Berman, et. al. 2015b).
Recent clinical trials have examined active immunotherapy against CRC. These strategies include vaccination with peptides derived from tumor-associated antibodies (TAAs), tumor cells, DC vaccines, and viral vectors.
Peptide vaccinations have been developed against the following antigens: carcinoembryonic (CEA), mucin 1 (EGFR), squamous-cell carcinoma antigen recognized on T-cells 3 (SART3); beta-human-chorionic-gonadotropin (beta hCG), Wilms tumor antigen 1, (WT1) Survivin-2B (MAGE3), ring-finger protein 43 (TOMM34), and mutated KRAS Patients in several phase I-II clinical trials showed antigen specific CTL responses and antibody production. “In contrast to the immunological response, many patients didn’t benefit from peptide vaccinations at the clinical level. (Koido, 2013; Miyagi, 2001; Moulton, 2002; Okuno, 2011).
Dendritic Cell Vaccines” are DCs that have been pulsed with TAA-derived Peptides, tumor cell Lysates, tumor RNA, or DC-tumor cells fusion products. Although many patients in phase II/III trials had specific immunological responses (Koido and colleagues, 2013), only a minority of them showed clinical benefits.
Esophageal Cancer
Click here to view the patent on Google Patents.