Invented by J. Ping Liu, University of Texas System
The market for nanowire magnets is still in its infancy, but it is expected to grow rapidly in the coming years. According to a report by MarketsandMarkets, the global market for nanowire magnets is projected to reach $1.4 billion by 2025, growing at a CAGR of 33.7% from 2020 to 2025.
One of the key drivers of this growth is the increasing demand for high-performance magnets in various industries, such as electronics, healthcare, and energy. Nanowire magnets offer several advantages over traditional magnets, including higher magnetic strength, greater stability, and improved efficiency.
There are several methods for making nanowire magnets, each with its own advantages and disadvantages. One of the most common methods is electrochemical deposition, which involves the use of an electric current to deposit metal ions onto a substrate, forming nanowires.
Another method is template-assisted synthesis, which involves the use of a template or scaffold to guide the growth of nanowires. This method allows for greater control over the size, shape, and orientation of the nanowires, making it ideal for applications that require precise control over the magnetic properties of the material.
Other methods for making nanowire magnets include chemical vapor deposition, physical vapor deposition, and sol-gel synthesis. Each of these methods has its own unique advantages and disadvantages, depending on the specific application and desired properties of the nanowire magnet.
Despite the potential of nanowire magnets, there are still several challenges that need to be addressed before they can be widely adopted in various industries. One of the biggest challenges is the scalability of the production process, as current methods for making nanowire magnets are often time-consuming and expensive.
Another challenge is the stability of the nanowire magnets, as they can be prone to oxidation and degradation over time. Researchers are currently working on developing new methods for stabilizing nanowire magnets, such as coating them with protective layers or using alloying techniques to improve their resistance to corrosion.
In conclusion, the market for nanowire magnets is poised for significant growth in the coming years, driven by the increasing demand for high-performance magnets in various industries. While there are still several challenges that need to be addressed, the potential of nanowire magnets to revolutionize the field of magnetism is undeniable, and researchers are working hard to overcome these challenges and unlock their full potential.

The University of Texas System invention works as follows
The present invention is a method of producing a high energy product by using ferromagnetic elements in 3D, such as nanowires. The magnets or high-energy products of the invention can achieve a high degree of magnetization, and retain their magnetic properties over a wider temperature range than other magnets. As an example, a product of high energy includes at least one of the materials A that is a nanowire formed through a solvothermal process. “A high-energy product can also include at the least one material A from the group consisting of essentially Fe, Co and Ni and at the least one other material B from the same group, both in the form an alloy of nanowires created by a chemical solvothermal process.

Background for Nanowire magnets and methods for making them
Permanent Magnets are made traditionally using rare earth minerals (REMs) in order to achieve high magnetization. Due to the diminishing resources, REMs have become more difficult and costly to obtain, making permanent magnets even more expensive. Permanent magnets have been in demand due to the dwindling REM resources. They are homogenized and used for all modern electronic and electrical devices. This trend has led to extensive research into the use of ferromagnets as an alternative to traditional REMs. Ferromagnetic materials have some advantages over REMs, such as a higher magnetization level and better thermal stability. Ferromagnetic material, though cheaper and more plentiful than REMs have failed to be an adequate replacement due to their high saturation magnetization and high Curie temperature. Low coercivity comes from low magneto-crystalline anisotropy.
The use of shape anisotropy in ferromagnets for coercivity development has been investigated previously. Alnico permanent magnets, which have been manufactured since the 1930s, are a good example. The Alnico microstructure consists of two phases: isolated needles with a FeCo-rich phase, and a matrix without ferromagnetic properties. Alnico’s performance is limited by the very low coercivity of these magnets (typical properties of Alnico commercial magnets are coercivity 1.5 kOe, and energy product 10 MGOe).
The recent research on magnetic nanoparticles has renewed interest in the development of high coercivity transition metal nanocrystals using shape anisotropy. Co and Fe-based ferromagnetic ferromagnetic ferromagnetic ferromagnetic ferromagnetic ferromagnetic nanwires and nanrods are produced using electrochemical deposition and chemical syntheses. “Aligned single-crystalline co nanorods have shown room-temperature coercivities up to 7.0kOe.
The coercivity of the magnets must be high, exceeding 10 kOe. This will allow them to become ideal building blocks in future thin-film, bonded and consolidated magnets that have high energy density.
The present invention provides magnets with high energy as an alternative to rare earth magnets. The invention provides a high-energy product or magnet that includes at least one magnet forming single-crystal nanowires which bond together to form the magnet or high-energy product. The magnetic element can be Fe, Co or Ni. The magnet material can be an alloy made of Fe and Co, where the ratio of Fe to Co by atomic percentage may range from 40:60 up to 70:30.
The magnet material can be developed into a product with high energy by first forming nanowires using a solvothermal method, and then forming resin bonded nanowires. In some cases more than 60% of the nanowires have their axes parallel. Individual nanowires can be either hexagonal or body-centered cubic crystals. Individual nanowires have a size in the range 1-200 nm, and a ratio of length to diameter between 10-50. In certain embodiments, nanowires can have an aspect of greater than 10 but less than 100. In certain instances, nanowires can have an aspect of greater than 10 but less than 30. In some cases, the nanowires have a larger diameter than 1 nm, but less than 50 nm. The nanowires can have a larger diameter than 10 nm, but less than 100 nm in some cases.
For example, the high-energy product contains at least one material selected from the group consisting of essentially Fe, Co, or Ni, in which the material is formed as nanowires by a chemical process involving solvothermal heat.
In addition, high-energy products include at least one of the materials A, selected from a group consisting of Fe Co and Ni and at least 1 of the materials B, selected from a group consisting of Fe Co and Ni. Material A and B are formed as an alloy of nanowires by a solvothermal chemistry process.
A method of manufacturing a high-energy product also includes the use of nanowires that are made from at least one material, A, selected from a group consisting essentially Fe, Co, or Ni, followed by a solvothermal chemistry process, which bonds the nanowires with a resin. In some cases, the nanowires can also include at least one other material B, selected from the group consisting of Fe, Co and Ni, so that the material A and the material B are an alloy.
Below, we describe the details of embodiments that are similar to those described above. “The present invention is described below in detail with reference to the accompanying illustrations.

While various embodiments of this invention will be discussed in detail, it is important to remember that many inventive concepts can be used in many contexts. These specific embodiments are only examples of how to make and use the invention. They do not limit the invention’s scope.
The high-energy products or magnets are made up of nanowires that have been bonded together. These nanowires are actually crystal structures in the magnet material. Magnet materials can be magnetic materials such as Co, or magnetic alloys such as Fe?Co. In the following section, you will find examples of magnet preparations for these two materials. Other alloys and magnetic materials can also be used. “Nanowires” includes nanorods, and other nanostructures that have the same characteristics as described below.
The high-energy products or magnets provided by the invention have a number advantages over known ferromagnets. The high energy magnets or products of the invention, for example, have better magnetization, and retain their magnetic properties better over a wide range of temperatures.
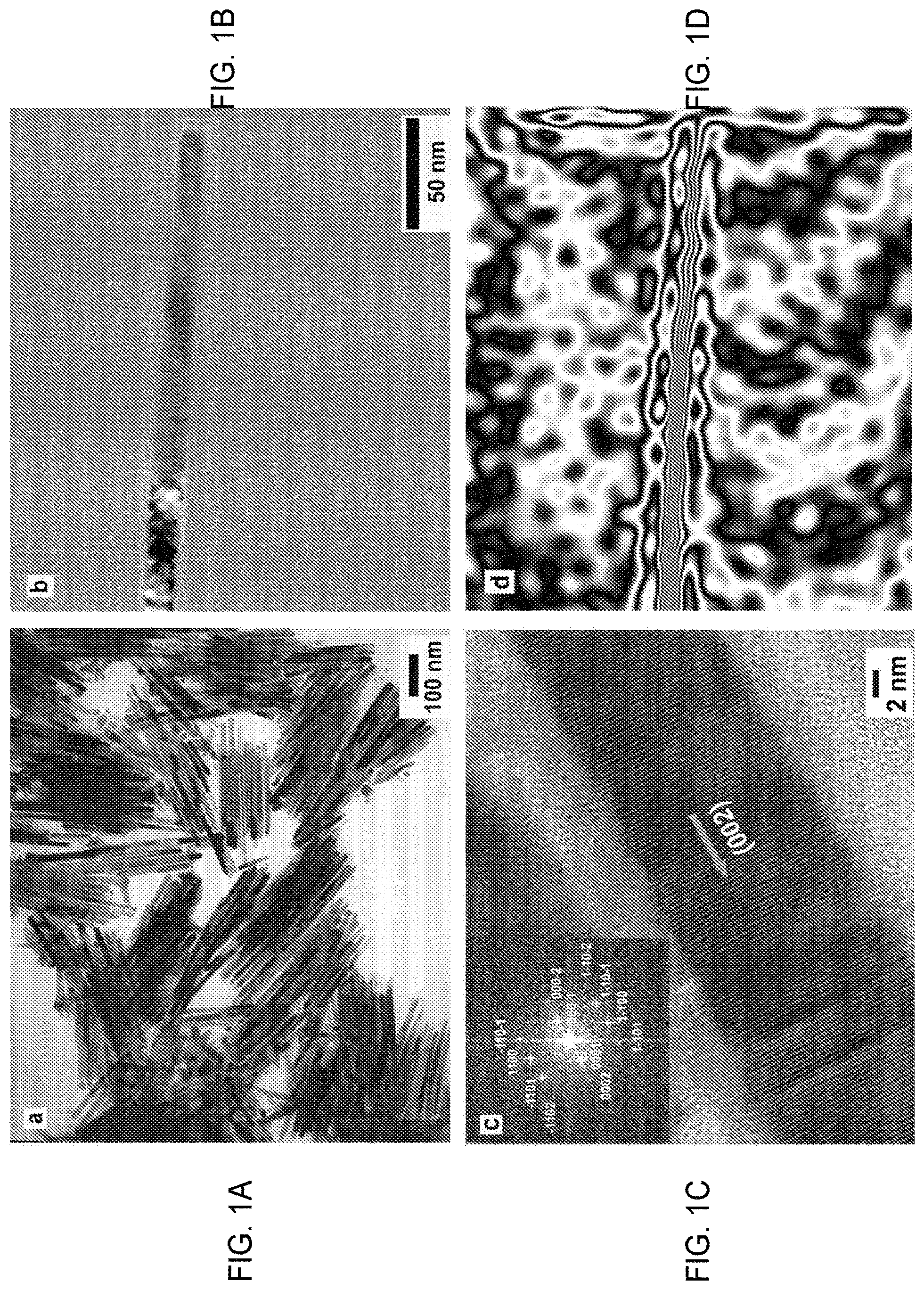
A material with a high aspect ratio nanocrystalline Co metal has exhibited a record high coercivity at room temperature. This was achieved through a chemical solvothermal synthesis that produced incredibly high energy in the material. The enhanced coercivity is a result of shape anisotropy, while the uniformity and orientation of single-domain nanocrystals play a key role in achieving high magnetic energies. As shown below, Co nanowires having a diameter of 15 nm, and an average of 200 nm, have a coercivity record of 10.3 kOe, at room temperature. This leads to an energy of 44 MGOe.
In a second example, single-crystal FeCo Nanowires were synthesized by reductively decomposing organometallic precursors in the presence surfactants. Monocrystalline FeCo Nanowires have a high magnetic coercivity of up to 1.2kOe when at room temperature. The effects of surfactant, Fe/Co precursors ratio, and heating rate on the magnetic properties, morphology and structure of the nanomaterial is described below.
Example 1 : Co Nanowire preparation
The following example will be presented as a non-limiting example for the preparation of Co nanowires in accordance with a particular embodiment of this invention.
The following materials can be used directly without further purification: CoCl2.6H2O, RuCl3 and methanol.
Examples of Co nanowires were developed using the above method and characterized in accordance with the following. Transmission electron microscopy images (TEM) were recorded using a JEOL EX 1200 electron microscope with an accelerating voltage 120 kV. Nanowires were produced by evaporating toluene on carbon-coated grids. Hitachi’s H-9500 high resolution TEM was used to obtain images with a 300 kV accelerated voltage. Lacey Carbon Grids were used to investigate high resolution TEM. The electron holography of a single cobalt nanowire image was digitally recorded at 200 kV accelerating voltage in a JEOL JEM-2100F LM field emission gun TEM with JEOL Biprism (0.6 mm in diameter, 180 u in rotation), within a remanent TEM field of about 4 Oe. The phase image reconstruction of the specimen gives a contoured picture of the magnetic flux.
Click here to view the patent on Google Patents.