Invented by Jennifer R. Cochran, Richard H. Kimura, Aron M. LEVIN, Leland Stanford Junior University
The market for engineered integrin binding peptide compositions is expected to grow significantly in the coming years. According to a report by MarketsandMarkets, the global peptide therapeutics market is projected to reach $48.5 billion by 2025, with a compound annual growth rate of 9.5%. This growth is driven by the increasing prevalence of chronic diseases, such as cancer and diabetes, which require targeted therapies that can be delivered directly to affected cells.
One of the key advantages of engineered integrin binding peptide compositions is their specificity. Unlike traditional drugs, which can have off-target effects and cause unwanted side effects, these peptides are designed to bind only to specific integrins, reducing the risk of adverse reactions. This makes them ideal for use in targeted drug delivery, where the goal is to deliver drugs directly to diseased cells while minimizing damage to healthy cells.
In addition to drug delivery, engineered integrin binding peptide compositions are also being used in tissue engineering. By incorporating these peptides into scaffolds or matrices, researchers can create materials that promote cell adhesion and growth, leading to the formation of new tissue. This has potential applications in regenerative medicine, where damaged or diseased tissue can be replaced with new, healthy tissue.
Another area where engineered integrin binding peptide compositions are being used is in diagnostics. By conjugating these peptides to imaging agents, researchers can create probes that can be used to detect specific integrins in vivo. This has potential applications in cancer imaging, where integrins are often overexpressed on the surface of tumor cells.
Overall, the market for engineered integrin binding peptide compositions is poised for significant growth in the coming years. With their specificity and versatility, these peptides have the potential to revolutionize drug delivery, tissue engineering, and diagnostics, leading to new and innovative therapies for a range of diseases. As research in this field continues to advance, we can expect to see even more exciting developments in the years to come.
The Leland Stanford Junior University invention works as follows
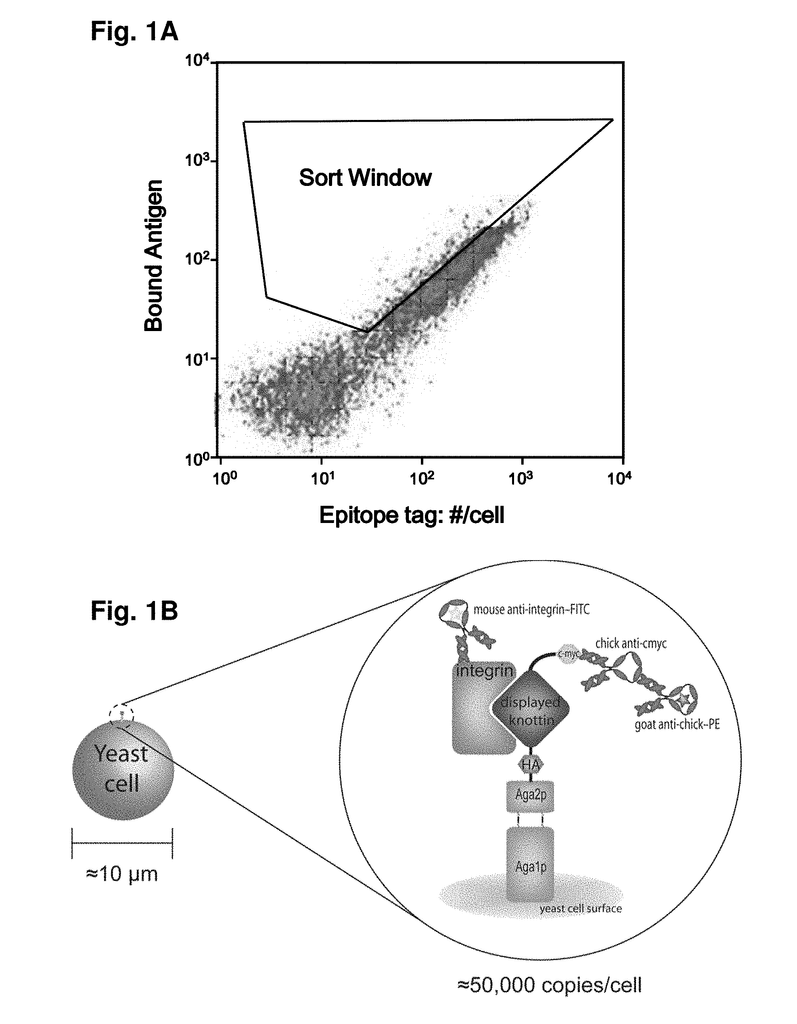
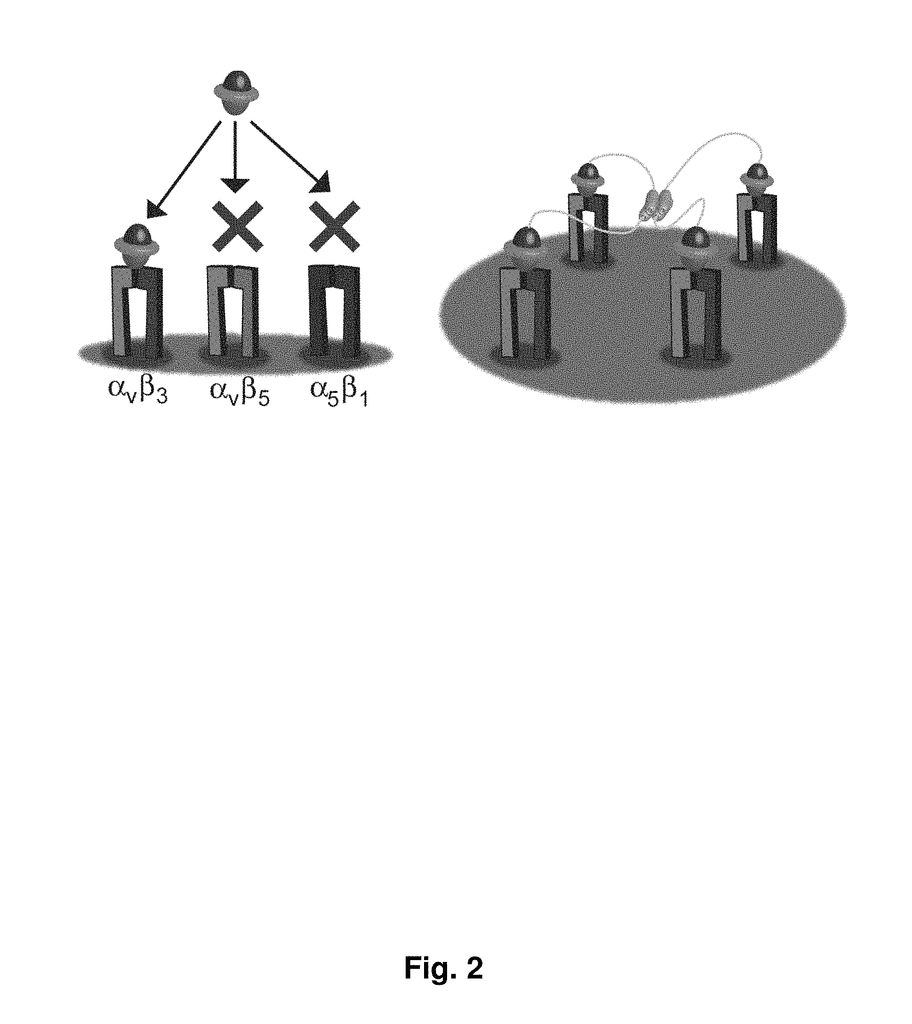
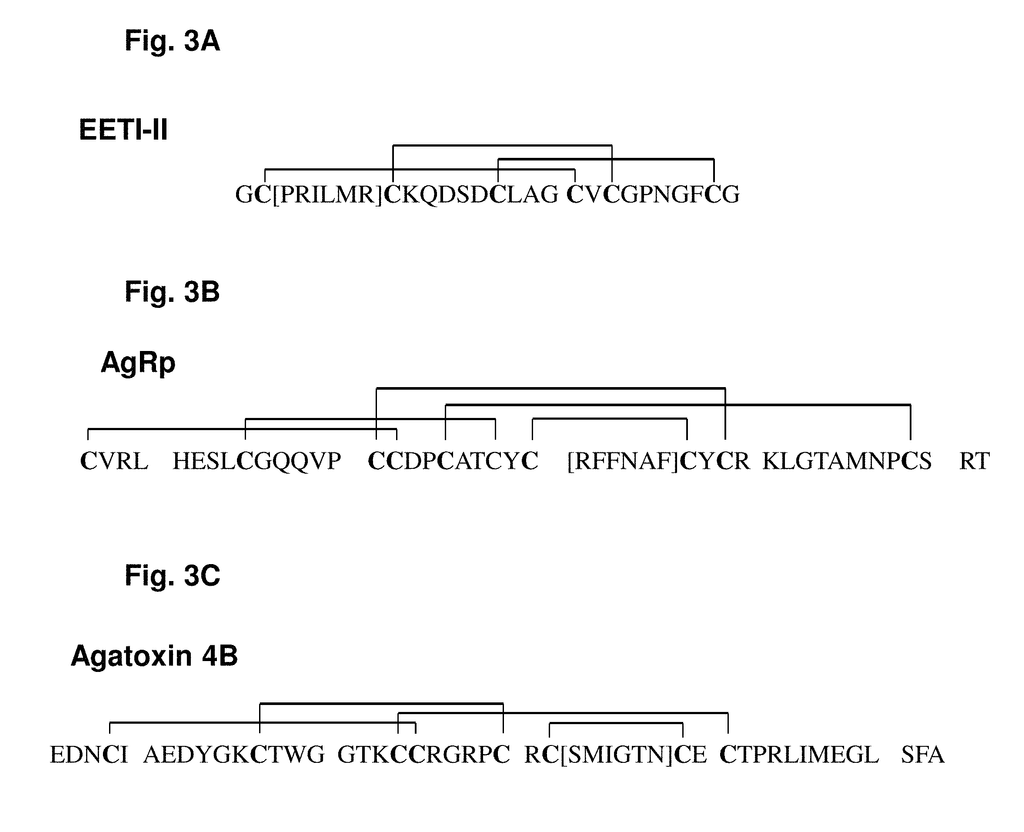
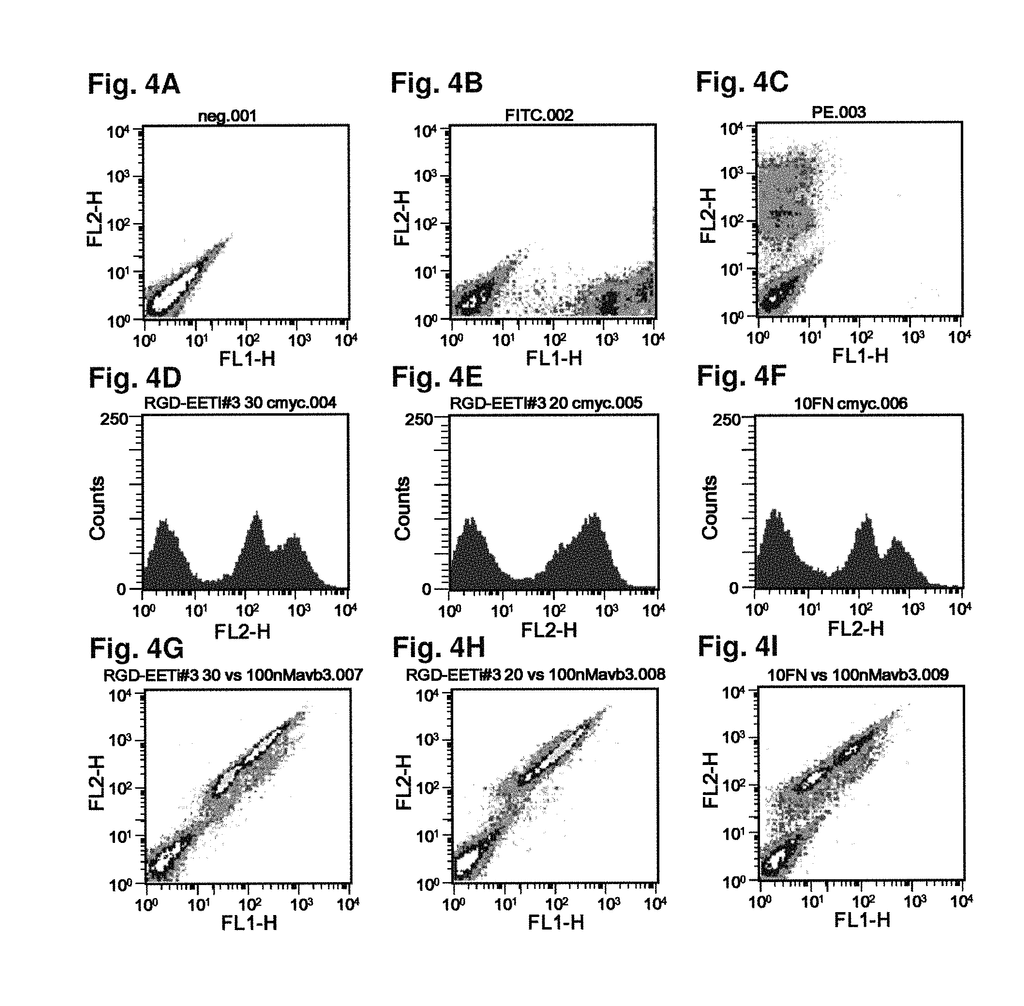
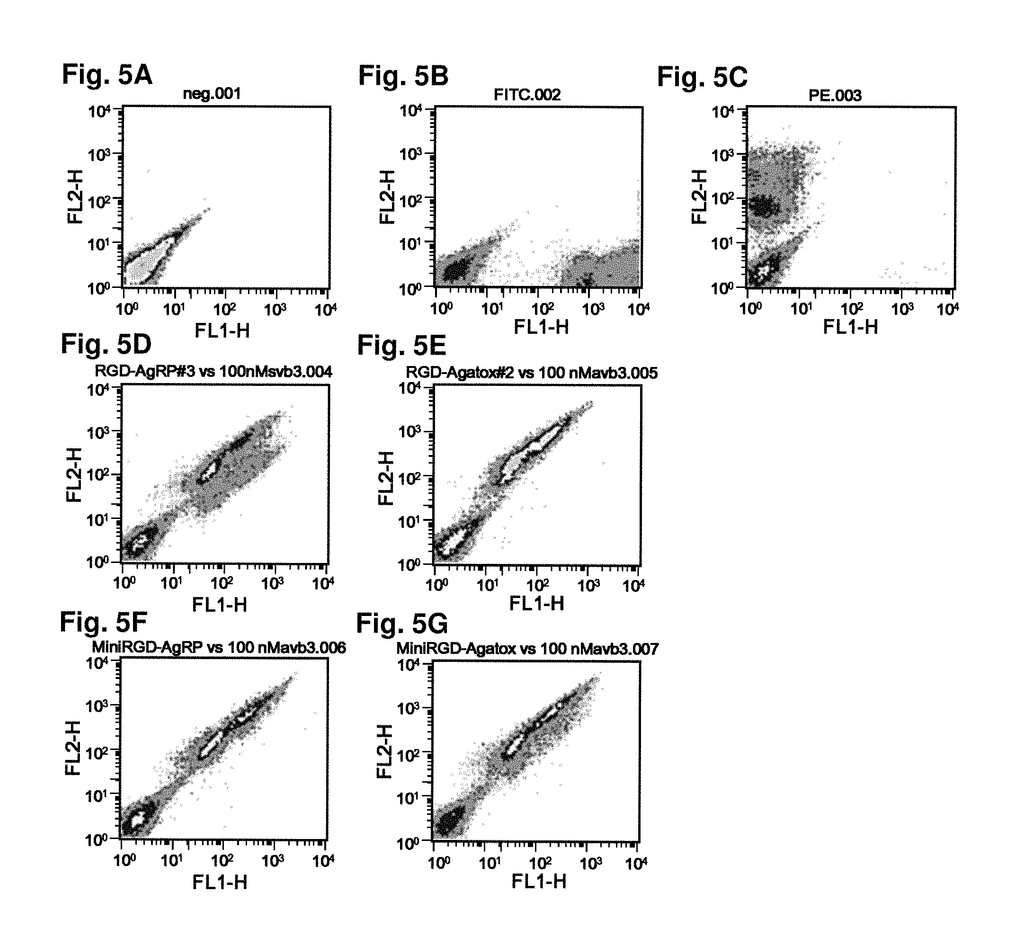
As imaging tissue, “engineered peptides that bind high affinity (low equilibriumdissociation constant (Kd),) to cell surface receptors for fibronectin (?)5?1) and vitronectin (?)3 and?v.5 integrins (?) are described as useful.” These peptides were created from a molecular scaffold that has had a subsequence with the RGD integrin binding motif. The subsequence, or RGD mimic, contains approximately 9-13 amino acid. It can also contain a variety other amino acids. Their sequence was determined through sequential rounds of selection (invitro evolution). A molecular scaffold should be preferably based upon a knottin. The EETI can tolerate mutations in other loops, and the present peptides could be used as imaging agents.
Background for Engineered integrin binding Peptide Compositions
Field of Invention
The invention pertains to engineered peptides and to the field peptides that bind to Integrins and, in particular, to integrin binding as it is related to cell growth.
Related Art
Integrins” are extracellular matrix adhesion protein families that can be noncovalently incorporated into? “Integrins are a family of extracellular matrix adhesion proteins that noncovalently associate into???? and?? Hynes, 1992; Luscinskas, Lawler, 1994). Cell adhesion is mediated through integrin-protein interactions and is responsible for cell motility. It also plays a role in survival and differentiation. Each ? Each???? and? subunit of the Integrin receptor contributes ligand binding to specificity and binding.
The Arg-Gly?Asp (RGD), a short peptide motif, can facilitate protein binding to many cell surface integrins (Pierschbacher & Ruoslahti 1984). These peptides serve two functions: they promote cell adhesion by being immobilized on a surface and inhibit cell adhesion if presented to cells in solution. The RGD sequence is found in adhesion proteins such as fibronectin and vitronectin. The RGD sequence is specific to approximately half of 20 known integrins. This includes the??5?1,?8?1,?v.3,???v.5,??v.6,.v.8, and.iib?3integrins. It also shows specificity to the???2?1,?3?1,?4?1 and.7?1integrins. The?v.3 integrin can bind to a wide range of RGD containing proteins, including fibrinogen, vitronectin and osteopontin (Ruoslahti 1996; Haubner et. al. 1997). While the?5??1 integrin has been only shown to bind with fibronectin (D’Souza et. al. 1991),
The affinity of the linear peptide RGD for integrins is much lower than that of the proteins it was derived from (Hautanen and al., 1989). This is due to the conformational speciality provided by folded protein domains that are not found in linear peptides. The preparation of cyclic RGD motif sequences, modification of residues flanking them, and the synthesis of small-molecule mimetics have led to an increase in functional integrin activity (reviewed by Ruoslahti, 1996, Haubner, et al. 1997 ).
The Xray crystal structure of the 10th Type III domain of Fibronectin (Dickinson and colleagues, 1994) and the NMR solution structures (Copie and coll., 1998), of the murine 9th & 10th Type III fibronection domains have been solved. These structures reveal that the GRGDSP (SEQID NO: 105) amino sequence is a type II?hairpin turn. This allows for interaction with integrin receivers.
According to NMR, short RGD peptides can also assume type II?-turns in aqueous solutions (Johnson and al., 1993). Conformation and stereochemistry about the RGD motif in the form of cyclic penta- and hexa-peptides, and disulfide-constrained peptides have been studied extensively (reviewed in (Haubner et al., 1997)). Combinations of unnatural and natural amino acids, peptidomimetics or disulfide bond flanking the RGD motif are necessary for creating high-affinity, biologically active,?-turn structures. Recent structure of an RGD ligand-receptor mimic bound to??v?3 (Xiong et. al. 2002) sheds light on the nature and function of this interaction. It also validates the body of work that has been done in relation to the ligand based design strategy.
Phage display technology was previously used to isolate cyclic propeptides that were specific to different integrin receptors. A random linear hexapeptide list was displayed on phage and was then panned with immobilized Integrin. The amino acid sequence CRGDCL was obtained (Koivunen, 1993). This peptide inhibited the attachment of fibronectin-expressing cells (Koivunen and al. 1993). It was 10 times more potent than RGD hexapeptides. The cyclic peptide also inhibited adhesion to cells mediated by integrins?v.1,?v.3, and??v?5. Another study used phage display to identify selective ligands for the?5??1,?v.3,?v.5, and???I1b?3 integrins using phage libraries that express cyclic peptides. (Koivunen, 1995). Each of the four integrins examined preferred RGD-containing sequences but preferred different ring sizes, flanking residues and RGD motif. ACRGDGWCG (SEQID NO: 2) was a cyclic peptide that was bound with high affinity the?5 and?1 integrins. The cyclic peptide ACDCRGDCFCG, which has two disulfide links (SEQ ID No: 3), was found to be 20 times more effective at inhibiting cell adhesion mediated via the?v.3 and??5 integrins than similar peptides that only have one disulfide bond and 200 times more potent than linear RGD.
Phage display can also be used to identify novel integrin binding motif from peptide library. The cyclic peptide, CRRETAWAC (SEQ Id NO: 4) was discovered from a random collection of heptapeptides phages with flanking cystine amino acids (Koivunen and al. 1994). The peptide was found to be specific for binding to?5?1 integrin and not the other?v.3 and??5 integrins. It also had an overlapping binding site with RGD sequence. The phage display library identified the peptide NGRAHA, SEQ ID NO. 5. However, it was later discovered that the receptor was actually aminopeptidase N and not integrins (Pasqualini, et. al. 2000). The synergistic binding site at the 10th domain (containing the sequence RNS), also increases RGD binding to?5?? integrin (Koivunen and Yoshizato 1995). The sequence PHSRN (SEQID NO: 6) from the 9th domain fibronectin increases the?5?1 Integrin binding to RGD peptides in fibronectin. (Aota and al., 1994). From a 15-mer library of phages, the sequence ACGSAGTCSPHLRRP was found (SEQ ID NO. 7) after it was panned using?v?3 Integrin. The sequence of vitronectin contains the SAGT (SEQID NO: 139), a tetrapeptide, which suggests that this could be an additional site for integrin binding and recognition (Healy et. al. 1995). The possibility of other synergy sites has been suggested (reviewed by Ruoslahti 1996), which suggests that random peptide library screening would be beneficial for finding integrin ligands that are not RGD.
Multiple RGD motifs in a single molecule have been shown to increase Integrin binding affinity and activity. Multiple studies have shown that RGD ligands clustered in multivalent groups within polymer-coated surfaces or beads can increase cell adhesion. This is due to either an increased local concentration or increased receptor/ligand avidity. (Miyamoto et al., 1995; Maheshwari et al., 2000; Pierschbacher et al., 1994; Shakesheff et al., 1998). Soluble RGD repeats incorporated into polypeptides (Saiki, 1997), or linked through a poly(carboxyethylmethacrylamide) backbone (Komazawa et al., 1993) have demonstrated an increased potential for inhibition of cancer metastasis compared to free peptide. Recent research has shown that soluble multivalent polymers (SEQID NO: 8) as well as copolymers (SEQID NO: 8) of GRGD and the?5?? synergy peptide, SRN, were synthesized by ring-opening metathesis (Maynard et. al. 2001). Homopolymers containing GRGD peptides (SEQ ID No: 8) were more powerful inhibitors of fibronectin cells adhesion (IC50=0.18 mM versus peptide only (IC50=1.08 mM). Maynard et. al. 2001. Heteropolymers containing both GRGD peptides (SEQ ID No: 8) as well as SRN peptides had an IC50 value of 0.03 mM. Multivalent hetero- and homo-oligomers (integrin peptides) showed increased inhibition of cell adhesion. However, multivalent frameworks can be used to improve affinity and efficacy.
Angiogenesis is the growth of blood vessels. It plays an important part in wound healing and development. Angiogenesis has been linked to proliferative diseases such as cancer and rheumatoid arthritis. It is therefore a promising target for therapeutic intervention. The ability of tumor cells to create new blood vessels to provide nutrients to tumor cells is crucial for their survival and growth. This new tumor vascularization allows for the release of tumor cells into the bloodstream, which can lead to metastases. Anti-angiogenic therapy can be achieved by inhibiting cell adhesion events within endothelial cells. Angiogenesis in vascular cells is dependent on the?v.3 and?v.5 integrins, as well as Kim et. al. (Kim, et. al. 2000). Brooks and his colleagues showed that the ‘v?3 integrin was expressed abundantly on blood vessels but not on epithelial or dermis cells. During angiogenesis, expression was also upregulated on vascular tissue (Brooks, et al. 1994). Kim et al. 2000 also showed that the?v.1 integrin is expressed in the tumor vasculature, breast, ovarian and prostate cancers. However, it was not found in normal adult tissues or blood vessels. Many tumor cells, including osteosarcomas and neuroblastomas as well as carcinomas of the breast, bladder, prostate, breast, and lung, are high-expressing?v.3 (and?v.5) integrins (reviewed by (Haubner and al., 1997). The aggressiveness of the tumor was also linked to the expression of?v.3 and??v.5 by neuroblastoma’s vascular endothelium (Erdreich?Epstein, 2000).
A monoclonal anti?v?3 antibody (LM609) has been shown to inhibit angiogenesis via fibroblast growth factor, tumor necrosis factors-a and human melanoma pieces (Brooks et. al., 1994). Vitaxin, a humanized version LM609 has been shown to inhibit tumor growth in animal models (Brooks et. al. 1995) and target angiogenic vessels (Sipkins et. al. 1998). Phase I clinical trials of Vitaxin have been completed in humans. It appears to be safe, and may play a role in disease stabilization (Gutheil and al. 2000). Another study showed that function-blocking monoclonal anti-?5??1 monoclonal antibodies inhibited cell adhesion and inhibited FGF-induced angiogenesis (Kim et. al., 2000). RGD peptides that are selective to?v (Pasqualini and al., 1997) and?5 integrins(Kim and al. 2000) are also relevant targets for imaging as well as therapeutic purposes. A Bacteriophage expressing an RGD peptide, (CDCRGDCFC), (SEQ ID No: 9) that has a high affinity to?v integrins (Ruoslahti 2000) was found to localize to tumors when injected into mice with tumors (Ruoslahti 2000). RGD containing both peptides or peptidomimetics has also been shown to be promising in cancer treatment. It binds to cell surface integrins, interfering with tumor blood supply and angiogenesis. The cyclic RGD Peptides were shown to inhibit?v.3 and??v.5 integrins and impair tumor growth in vivo (Brooks and al. 1994; Buerkle and al. 2002). The cyclic peptide (SEQ ID No: 10) was also shown to induce?v.3-mediated apoptosis (Chatterjee and al. 2000; Buerkle et. al. 2001). The nonpeptide antagonist SJ749 and the cyclic peptide antagonist, CRRETAWAC, inhibited?5?1-mediated cell adhesion via fibronectin (SEQ ID No: 11). Kim et al., 2000. The integrin inhibitors appear to have no effect upon normal vessels and seem to act by inducing angiogenesis in newly formed endothelial cell populations (Brooks and al. 1994) and interfering in the function of metalloproteinase enzymes necessary for cellular invasion (Brooks and al. 1996).
Radiolabeled integrin inhibitors are useful for tumor targeting and imaging. For clinical applications of integrin inhibitors, it is important to use noninvasive methods to quantify and visualize integrin expression in vivo (Brower 1999). Radioiodinated cyclic RGD Peptides were the first to exhibit high specificity and affinity in vitro and in-vivo for?v3 integrins. However, they had rapid excretion and accumulation in liver and intestines which limited their use (Haubner and colleagues, 1999). These peptides were modified with a sugar moiety to reduce their liver uptake and increase their accumulation in vivo in?v3-expressing tumors (Haubner, et al. 2001). The noninvasive imaging using an 18F-labeled glycoRGD peptide via positron emission tomography showed receptor-specific binding and high tumor-to-background ratios in vivo. This suggests that it is suitable for?v3 quantification and treatment (Haubner, et al. 2001). RGD peptides that are coupled with chelating agents can be radiolabeled using 111In, 120I, 90Y, and 177Lu. This increases their potential for tumor imaging as well as radionuclide treatment (van Hagen, et al. 2000). Integrin-specific antibodies are also useful in imaging applications. For detailed imaging of rabbit carcinomas, paramagnetic liposomes coated in the anti?v.3 integrin antibody LM609 could be used (Sipkins et. al. 1998). These small integrin binding protein would be well-suited to being coupled to a wide range of radionuclides or chemotherapeutic agents.
Patents & Publications
Ruoslahti and colleagues have received a number of patents in relation to RGD peptides. U.S. Pat. No. No. 5,695,997 entitled?Tetrapeptide? 5,695,997, entitled?Tetrapeptide,? Further, the patent includes an embodiment in which X can be any amino acid and the cell attachment activity of the peptide is less than 31 amino acids.
Similarly, U.S. Pat. No. 4,792,525 relates to a substantially pure peptide including as the cell-attachment-promoting constituent the amino acid sequence Arg-Gly-Asp-R wherein R is Ser, Cys, Thr or other amino acid, said peptide having cell-attachment promoting activity, and said peptide not being a naturally occurring peptide.
U.S. Pat. No. No.
U.S. Pat. No. 5,536,814, to Ruoslahti, et al., entitled ?Integrin-binding peptides,? issued Jul. 16 1996. Contains a purified synthetic propeptide containing certain amino acid sequences.
U.S. Pat. No. No. These RXD analogues and DGR analogs are called “RXD surrogates.
US 2005/0164300 to Artis, et al., published Jul. 28. 2005, entitled “Molecular scaffolds for kinase-ligand development.” This article identifies molecular scaffolds that can help identify and develop ligands for one or more kinases (e.g. the PIM kinases PIM-1, PIM-2 and PIM-3).
Click here to view the patent on Google Patents.