Invented by X. Christopher Yu, Atia Naim, Christopher A. Teske, Martin Vanderlaan, Genentech Inc
Monoclonal antibodies are laboratory-produced molecules that can mimic the immune system’s ability to fight off harmful pathogens. These antibodies are designed to target specific proteins or cells in the body, and they have been used to treat a variety of diseases, including cancer and autoimmune disorders. In the case of Alzheimer’s disease, monoclonal anti-abeta antibodies are designed to bind to and remove Abeta plaques from the brain.
The market for compositions containing purified monoclonal anti-abeta antibodies is driven by the increasing prevalence of Alzheimer’s disease. According to the Alzheimer’s Association, more than 6 million Americans are living with Alzheimer’s disease, and this number is expected to triple by 2050. There is currently no cure for Alzheimer’s disease, and existing treatments only provide temporary relief of symptoms. Monoclonal anti-abeta antibodies represent a promising new approach to treating the disease, and they are expected to become a major player in the Alzheimer’s disease market.
Several pharmaceutical companies are currently developing monoclonal anti-abeta antibodies for the treatment of Alzheimer’s disease. Biogen’s Aducanumab was recently approved by the FDA, and other companies, such as Eli Lilly and Roche, are also developing similar drugs. These companies are investing heavily in research and development to bring these drugs to market, and the market for compositions containing purified monoclonal anti-abeta antibodies is expected to grow significantly in the coming years.
In addition to the development of new drugs, the market for compositions containing purified monoclonal anti-abeta antibodies is also being driven by the increasing demand for personalized medicine. Personalized medicine involves tailoring treatments to individual patients based on their genetic makeup, lifestyle, and other factors. Monoclonal anti-abeta antibodies are well-suited for personalized medicine because they can be designed to target specific types of Abeta plaques in the brain. This approach could lead to more effective treatments for Alzheimer’s disease and other neurodegenerative disorders.
In conclusion, the market for compositions containing purified monoclonal anti-abeta antibodies is a rapidly growing industry that is expected to become a major player in the Alzheimer’s disease market. With the increasing prevalence of Alzheimer’s disease and the growing demand for personalized medicine, the development of new drugs and treatments is essential. Monoclonal anti-abeta antibodies represent a promising new approach to treating Alzheimer’s disease, and they are expected to have a significant impact on the healthcare industry in the coming years.
The Genentech Inc invention works as follows
The purified recombinant peptides, including therapeutic antibodies and antibodies, are isolated from Chinese Hamster Ovary host cells. Methods of making and utilizing such polypeptides is also provided.
Background for Compositions containing purified monoclonal anti-abeta antibodies
There are a number of drugs on the market and in development to treat asthma and other respiratory diseases. IL-13 is one of the targets in asthma treatment. IL-13, a pleiotropic TH2 cytokine, is produced by activated cells of the T-cell, NKT cell, basophils and mast cells. It has been implicated strongly in asthma pathogenesis in preclinical model. IL-13 inhibitors, such as anti-IL-13 antibody, have been previously described. Certain antibodies were also developed for human therapeutics. Recent studies have demonstrated the clinical effectiveness of monoclonal anti-IL-13 antibodies in treating asthma. (See, e.g. Corren et. al., 2011 N. Engl. J. Med. 365, 1088-1098; Gauvreau et al., 2011, Am. J. Respir. Crit. Care Med. Care Med. Immunol. 130, 829-42; Webb, 2011, Nat Biotechnol 29, 860-863). Lebrikizumab is a humanized IgG4 antigen that neutralizes IL-13. It improved lung function for asthmatics who had symptoms despite being treated with inhaled steroids and a beta2-adrenergic agonist. J Med. 365, 1088-1098).
In addition, IL-13 is implicated in a number of other allergic and fibrotic conditions.” For example, IL13 can be a mediator of a variety of diseases, including but not limited to allergic asthma, nonallergic asthma, atopic dermatitis (atopic dermatitis), allergic conjunctivitis (atopic dermatitis), eczema and urticaria. It also mediates conditions such as chronic obstructive pulmonary disorder, ulcerative colitis and RSV infection.
It is essential that any residual impurities from the manufacturing and purification processes are removed from the biological product. These components include host cell proteins, culture medium proteins and immunoglobulin affinity-ligands. These host cell impurities include process-specific host cell proteins (HCPs), which are process-related impurities/contaminants in the biologics derived from recombinant DNA technology. HCPs may be present in very small amounts (in parts per million or nanograms/milligrams of the intended recombinant proteins), but it is acknowledged that HCPs should be reduced. For example, the U.S. Food and Drug Administration (FDA) requires that biopharmaceuticals intended for in vivo human use should be as free as possible of extraneous impurities, and requires tests for detection and quantitation of potential contaminants/impurities, such as HCPs.
Procedures to purify proteins from cell debris depend at first on the site where the protein was expressed.” Some proteins are released directly into the growth medium from the cells, while others are produced intracellularly. The first step in a purification procedure for the latter proteins is lysis of cells. This can be achieved by various methods including mechanical shears, osmotic treatments, or enzyme treatments. This disruption allows the contents of the cells to be released into the homogenate. It also produces small subcellular fragments, which are hard to remove because they are so small. These are usually removed using centrifugation and filtration. This problem also occurs with directly secreted protein due to the death of cells naturally and the release of intracellular host-cell proteins during the production of proteins.
Once the solution containing the desired protein is obtained, it is separated from other proteins produced by cells using a variety of chromatography methods. These techniques typically separate proteins based on their size, hydrophobicity or charge. Each of these techniques can be tailored to the specific protein by using a variety of chromatography resins. Each of these methods involves causing proteins to either move at different speeds down a column to achieve a physical separation which increases as they progress down the column or to adhere to the separation medium and be differentially eluted using different solvents. In some cases, impurities adhere to a column and the desired protein does not. This is called the “flow-through.
The “Ion-Exchange Chromatography” is named after the counterion that can be exchanged. It is used to purify ionizable molecule. Ionized molecule are separated based on non-specific electrostatic interactions of their charged groups and oppositely charged molecules attached onto the solid phase support matrix. This results in the retardation of those ionized molecules that interact with the solid phase more strongly. The affinity of each ionized type molecule for the matrix varies depending on the number of groups charged, the charge per group and the nature and composition of molecules that are competing to interact with the charged matrix. Ion-exchange separation of different molecule types is possible due to these differences. In ion exchange purification, proteins from mammalian cells are mixed with a column of ion-exchange ions. After the non-binding molecules have been washed out, the conditions are changed, for example by changing the pH, the counter ion’s concentration, and so on in a step-mode or gradient mode, to release the non-specifically retarded or ionized proteins of interest from the solid phase and separate it from other proteins with different charge characteristics. In anion exchange chromatography, an anionic molecule competes with the negative counter-ion to interact with a positively charged matrix molecule at a specific pH and separation conditions. Cation exchange chromatography, on the other hand, involves a cationic molecular of interest competing with the positive counter-ion to interact with a negatively-charged molecule attached the solid phase matrix. This occurs at a specific pH and in the context of a certain separation process. In mixed mode ion-exchange chromatography, also known as multimodal ion-exchange chromatography, cation exchange and anion-exchange chromatographic media are used in the same step. In particular, “mixed mode” refers to a solid phase support matrix that is covalently attached with a mixture of cation exchange, anion exchange and hydrophobic interaction moieties. “Mixed mode” refers to the solid phase support matrix that is covalently attached with a mixture of anion exchange, cation-exchange, and hydrophobic interactions moieties.
The “Hydroxyapatite Chromatography of Proteins” involves the non-specific interactions of charged amino or carboxylate group of a proteins with oppositely charged groups in the hydroxyapatite. The pH of the buffer controls the net charge on both the hydroxyapatite as well as the protein. Elution is accomplished by displacing the non-specific protein-hydroxyapatite pairing with ions such as Ca2+ or Mg2+. “Phosphates, for example, are used to displace negatively-charged protein groups. This results in a protein that is net-negatively charged.
Hydrophobic Interaction Chromatography (HIC), is used to purify and separate molecules such as proteins based on their differences in surface hydrophobicity. Hydrophobic group of a proteins interacts non-specifically to hydrophobic group coupled with the chromatography matrix. Differential retardation on a HIC-column is caused by differences in the number and type of hydrophobic groups of a protein.
Affinity Chromatography, which uses a spatially complementary interaction between a protein to purify and an immobilized capture agents, is a standard option for purifying some proteins such as antibodies. For example, Protein A is an adsorbent that can be used for affinity chromatography for proteins such as antibodies. “Protein A is a cell wall protein of 41 kD from Staphylococcus aurias that binds to antibodies with high affinity (10?8M).
Purification of recombinant proteins is usually performed by using either bind-and-elute (B/E), or flow through (F/T) (flow-through) chromatography. Below, we describe them briefly.
Bind-and-Elute Chromatography” (B/E). In B/E, the product is loaded in order to maximize the dynamic binding capacity (DBC). Then wash and elution are optimized to achieve maximum purity of the product.
Various B/E method for use with Protein A affinity chromatography including various intermediate washing buffers have been described.” U.S. Pat. Nos. Nos. 6,127,526 et 6,333,398 describe a step of intermediate washing during Protein A chromatography, using hydrophobic electrodes, such as tetramethylammonium (TMAC) chloride and tetraethylammonium (TEAC), in order to remove contaminants but not immobilized Protein A, or the protein that is of interest bound to the Protein A columns. U.S. Pat. No. “6,870,034 describes methods and wash buffers to be used with protein A affinity chromatography.
Flow Through Chromatography” (F/T). Using F/T, it is possible to identify load conditions where impurities bind strongly to the chromatography materials while the product passes through. F/T Chromatography allows for high load densities in standard monoclonal antibodies (MAbs).
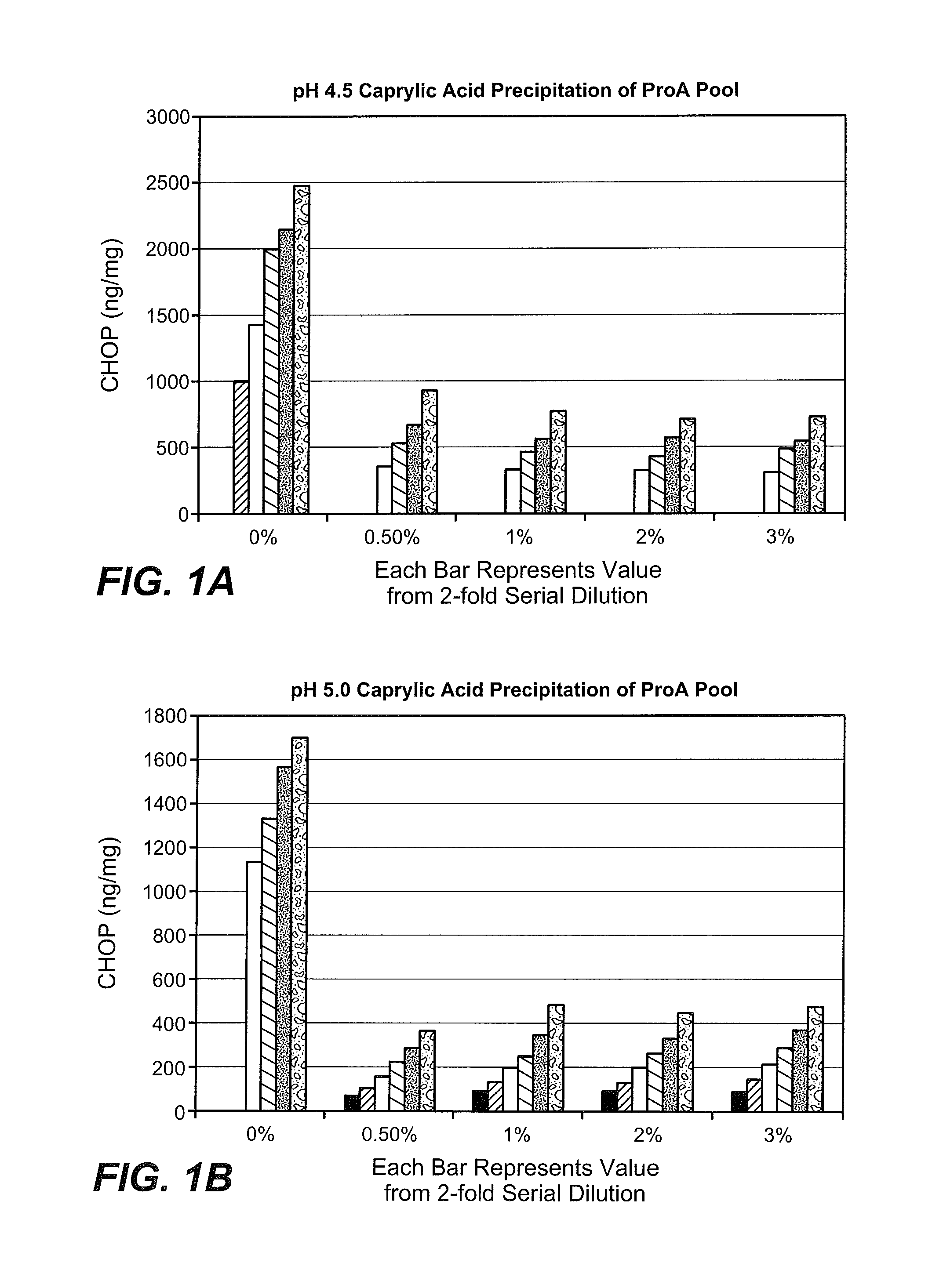
In a total CHOP ELISA, we found that a CHOP enzyme, phospholipase B like 2, was present in excess to the antibodies available in recombinant anti IL13 MAb and certain other recombinant peptides made in CHO cell cultures. As used herein, ?PLB2? As used herein,?PLB2? Both?PLBL2? and?PLBD2?” Both?PLBD2? and?PLB2? are interchangeable terms that refer to?phospholipase-like enzyme 2? It is also known as ‘phospholipase-domain-like 2,’ or PLBL2. Certain scientific publications on PLBL2 include Lakomek, K. et al., BMC Structural Biology 9:56 (2009); Deuschi, et al., FEBS Lett 580:5747-5752 (2006). The pre-pro enzyme PLBL2 has a parent MW of approximately 66,000. During post-translational modifications, the initial leader sequence is removed. Potential 6 mannose-6 phosphate (M-6P) groups can then be added. M-6P is a targeting modifcation that directs the enzyme to the Lysosome through the M-6P receptor. PLBL2 has six cysteines. Two of them have free sulfhydrals and four have disulfide bond. In acidic conditions, PLBL2 fragments with 32,000 and 45, 000 MW are further cut. This cleavage, like other lysosomal enzymatic cleavages, is an activating process, which allows the substrate to reach the active site.
There is a homology of 80% between the hamster- and human-derived forms of PLBL2. It is believed that the enzyme’s activity is to cleave fatty acids from phospholipids, which make up cell membranes. Other phospholipases have different substrate specificities. Microorganisms have similar enzymatic activity, which is often a virulence-factor. The enzymatic activity of microorganisms is similar, but the proteins that generate this activity are different. There is also a low degree of sequence homology in PLBL2 enzymes from mammalian and microbial sources. As a result of substrate hydrolysis, phospholipases can produce free fatty acid (FFA). Free fatty acid is a possible immune-signaling component. Dehydrogenation transforms FFA into arachadonic acids which may participate in inflammation cascades that involve eicosanoids.
We developed reagents, kits, and methods for the sensitive and quantitative measurement of PLBL2 in anti-IL-13 MAb preparations as well as other recombinant products produced in CHO cell lines. The Examples below, as well as in U.S. Patent No. Provisional Patent Applications Nos. 61/877.503 and 61/991.228. There was also the challenge of developing a robust and efficient large-scale process for purifying anti-IL13 MAb and other recombinant peptide products, resulting in MAb with sufficient purity (including the removal of PLBL2), for human therapeutic uses, including late-stage commercial and clinical use. The invention described in this document meets some of the needs above and also provides other benefits.
All references, including publications and patent applications, are incorporated in their entirety by reference.
The invention is based at least in part on the development and improvement of purification processes for recombinant peptides made in Chinese Hamster Ovary (CHO) cell lines that produce purified products with significantly reduced levels of hamster-PLBL2. The invention includes recombinant polypeptides that are purified using the invention’s methods, such as therapeutic antibodies like an anti-IL13 antigen. These products may be less immunogenic when administered to humans.
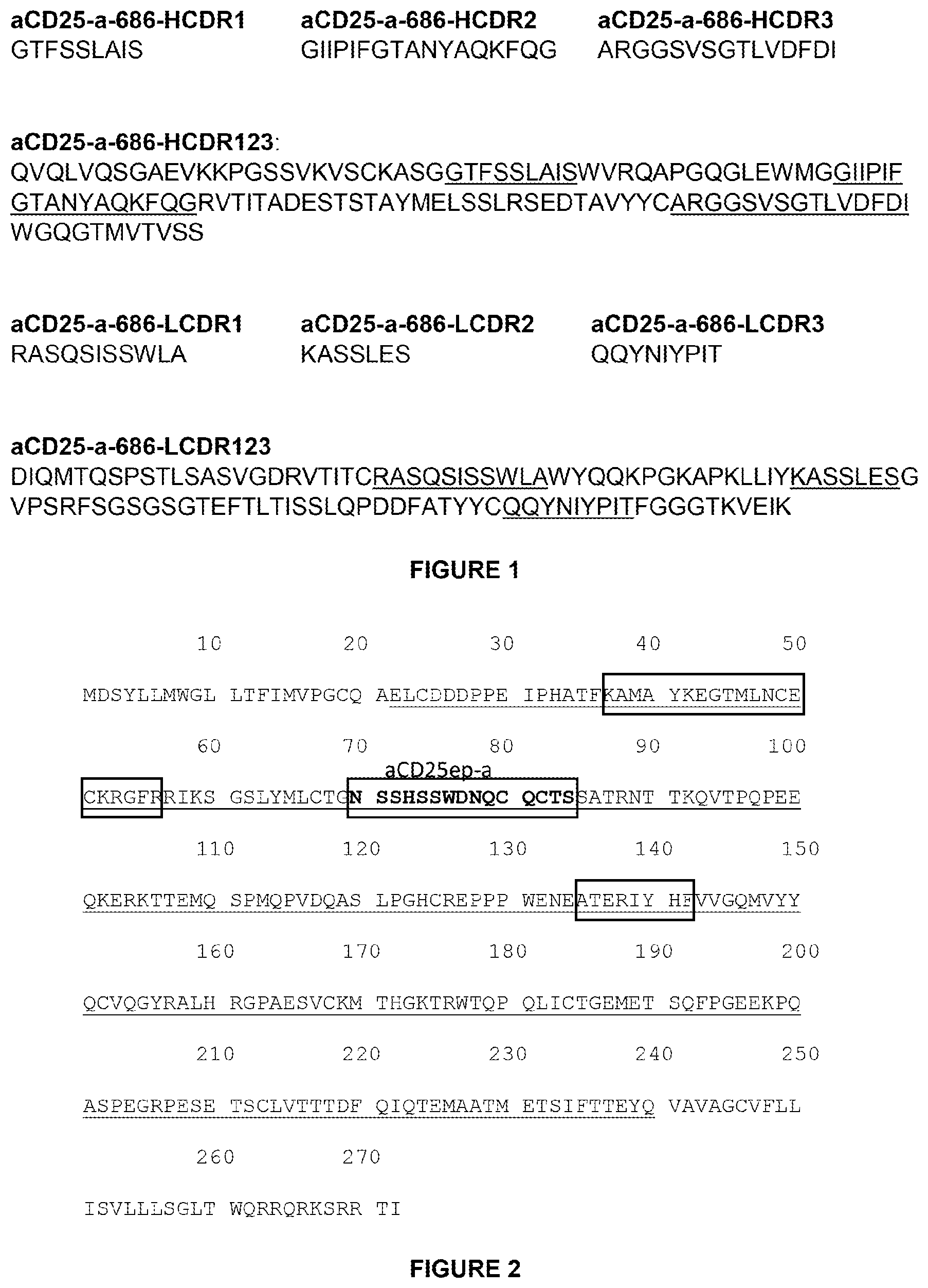
In one aspect, compositions containing an anti-IL13 polyclonal monoclonal antigen purified from CHO cell lysates containing the anti-IL13 antigen and a residual quantity of hamster PLBL2 is provided. In some embodiments, hamster-PLBL2 amounts are less than 20ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 15ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 10ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 8ng/mg. In certain embodiments the amount of Hamster PLBL2 less than 5ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than three ng/mg. In certain embodiments the amount of Hamster PLBL2 in certain embodiments is less than 2ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 1ng/mg. In some embodiments, hamster-PLBL2 amounts are less than 0.5ng/mg. In certain embodiments the amount of hamster-PLBL2 is 0.5ng/mg to 20ng/mg or 0.5ng/mg to 15ng/mg or 0.5ng/mg to 8ng/mg or 0.5ng/mg to 5ng/mg or 0.5ng/mg to 3ng/mg or 0.5ng/mg & 2ng/mg or 0.5ng/mg & 1ng/mg or 0.5ng/mg/mg/mg/mg/mg/mg/mg/mg/mg/mg/ml/mg/mg/mg/mg/mg/mg/mg/mg/mg/m/mg/mg/mg/mg/mg/mg/mg/mg/mg/mb/mg/mg/mg/mg/mg/mg/ and??????????????????????????????????????????????????????????? In certain embodiments the anti-IL13 antibodies comprises three heavy chains CDRs. CDR-H1 has the amino acid sequence SEQ ID No. CDR-H2 has the amino acid code of SEQID NO. CDR-H3 has the amino acid sequence SEQ ID No. Three light-chain CDRs and CDR-L1, which has the amino acid sequence SEQ ID No. CDR-L2 has the amino acid sequence SEQ ID No. CDR-L3 has the amino acid sequence SEQ ID No. : 6. In certain embodiments the anti-IL13 antibodies contain a variable heavy chain region with the amino acid sequence SEQ ID No. : 7. In certain embodiments the anti-IL13 antibodies contain a variable light chain region with the amino acid sequence SEQ ID No. : 9. In certain embodiments the anti-IL13 antibodies contain a heavy chain with the amino acid sequence SEQ ID No. : 10. In certain embodiments the anti-IL13 antibodies contain a lightchain with the amino acid sequence SEQ ID No. : 14. In certain embodiments the anti-IL13 antibodies include a heavy chain region with the amino acid sequence SEQ ID No. The anti-IL13 antibody may include a heavy chain variable region with the amino acid sequence SEQ ID No. : 9. In certain embodiments the anti-IL13 antibodies comprise a heavy chains having the amino acids sequence of SEQID NO. The anti-IL13 antibody may include a heavy chain with the amino acid sequence SEQ ID No. : 14. In certain embodiments the amount of hamster-PLBL2 is quantified by an immunoassay, or mass spectrometry. In certain embodiments the immunoassay used is a total Chinese hamster ovary ELISA, or a hamster PLBL2 ELISA. In certain embodiments the mass spectrometry test is LC/MS/MS.
In another aspect, anti IL13 monoclonal antibodies are isolated and purified by a method that includes a hydrophobic interactions chromatography step (HIC). In certain embodiments the purified preparation contains the anti-IL13 antibodies and a residual quantity of hamster PLBL2. In certain embodiments the amount of Hamster PLBL2 in a purified preparation is less than 20ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 15ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 10ng/mg. In certain embodiments the amount of Hamster PLBL2 in a given sample is less than 8ng/mg. In certain embodiments the amount of Hamster PLBL2 less than 5ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than three ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 3 ng/mg. In certain embodiments the amount of Hamster PLBL2 in these hamsters is less than 0.5 ng/mg. In some embodiments, hamster-PLBL2 amounts are less than 0.5ng/mg. In certain embodiments the amount of hamster-PLBL2 is less than 0.5ng/mg. In some embodiments, PHENYL SEPHHAROSE is used in the HIC step. 6 Fast Flow resin (High Sub). In certain embodiments the HIC step includes operating a column containing resin in flow-through mode. In certain embodiments the HIC step includes an equilibration and wash buffer. Each of the wash and equilibration are 50 mM sodium-acetate pH 5. In certain embodiments the flow through is monitored using absorbance at 280nm and the flow through is collected between 0.5OD to 1.5OD. In some embodiments, flow-through can be collected up to 8 column volumes. In certain embodiments the process includes an affinity chromatography. In some embodiments, affinity chromatography can be protein A chromatography. In certain embodiments the process includes an additional ion-exchange chromatography step. In certain embodiments the ion-exchange chromatography comprises anion-exchange chromatography. In certain embodiments the anti-IL13 antibodies comprises three heavy chains CDRs. CDR-H1 has the amino acid sequence SEQ ID No. CDR-H2 has the amino acid code of SEQID NO. CDR-H3 has the amino acid sequence SEQ ID No. Three light-chain CDRs and CDR-L1 with the amino acid sequence SEQ ID No. CDR-L2 has the amino acid sequence SEQ ID No. CDR-L3 has the amino acid sequence SEQ ID No. : 6. In certain embodiments the anti-IL13 antibodies contain a variable heavy chain region with the amino acid sequence SEQ ID No. : 7. In certain embodiments the anti-IL13 antibodies contain a variable light chain region with the amino acid sequence SEQ ID No. : 9. 9. In certain embodiments the anti-IL13 antibodies contain a heavy chains with the amino acid sequence SEQ ID No. : 10. In certain embodiments the anti-IL13 antibodies contain a lightchain with the amino acid sequence SEQ ID No. : 14. In certain embodiments the anti-IL13 antibodies include a heavy chain region with the amino acid sequence SEQ ID No. The anti-IL13 antibody may include a heavy chain variable region with the amino acid sequence SEQ ID No. : 9. In certain embodiments the anti-IL13 antibodies comprise a heavy chains having the amino acids sequence of SEQID NO. The anti-IL13 antibody may include a heavy chain with the amino acid sequence SEQ ID No. : 14. In certain embodiments the amount of hamster-PLBL2 can be quantified by an immunoassay, or mass spectrometry. In certain embodiments the immunoassay used is a hamster PLBL2 ELISA, or a total Chinese hamster ovary ELISA. In certain embodiments the mass spectrometry test is LC/MS/MS.
Purified anti-IL13 monclonal antibodies isolated from CHO cell are also provided in another aspect. In certain embodiments the antibody preparation can be purified using a process that includes a first Protein-A affinity chromatography, a second Anion Exchange Chromatography step and a final Hydrophobic Interaction Chromatography (HIC). In certain embodiments the amount of hamster-PLBL2 is lower than 20 ng/mg. In certain embodiments the amount of Hamster PLBL2 in a given sample is less than 15ng/mg. In certain embodiments the amount of Hamster PLBL2 in a given hamster is less than 10ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 8ng/mg. In certain embodiments the amount of Hamster PLBL2 less than 5ng/mg. In certain embodiments the amount of Hamster PLBL2 in a given sample is less than three ng/mg. In certain embodiments the amount of Hamster PLBL2 in the blood is less than 3 ng/mg. In certain embodiments the amount of Hamster PLBL2 in a given sample is less than one ng/mg. In some embodiments, hamster-PLBL2 amounts are less than 0.5ng/mg. In certain embodiments the amount of hamster-PLBL2 is within a range of 0.5ng/mg to 20ng/mg or 0.5ng/mg to 15ng/mg or 0.5ng/mg to 8ng/mg or 0.5ng/mg to 5ng/mg or 0.5ng/mg upto 3ng/mg or 0.5ng/mg between 2ng/mg or 0.5ng/mg between 1ng/mg or In some embodiments, MABSELECTSURE is used in the affinity chromatography. In certain embodiments, the affinity chromatography step includes MABSELECT SURE? Q SEPHAROSE Fast Flow is used in the anion exchange chromatography step, while PHENYL SEPHHAROSE? 6 Fast Flow. In certain embodiments the affinity chromatography includes operating a MABSELECT? The anion exchange chromatography comprises operating a Q SEPHHAROSE? Fast Flow resin containing column in binding-elute mode. The HIC step involves operating a PHENYL SEPHHAROSE? Fast Flow resin-containing column (High Sub), in flow-through. In certain embodiments the anti-IL13 antibodies contain three heavy chains CDRs. CDR-H1 has the amino acid sequence SEQ ID No. CDR-H2 has the amino acid code of SEQID NO. CDR-H3 has the amino acid sequence SEQ ID No. Three light-chain CDRs and CDR-L1, which has the amino acid sequence SEQ ID No. CDR-L2 has the amino acid sequence SEQ ID No. CDR-L3 has the amino acid sequence SEQ ID No. : 6. In certain embodiments the anti-IL13 antibodies contain a variable heavy chain region with the amino acid sequence SEQ ID No. : 7. In certain embodiments the anti-IL13 antibodies contain a variable light chain region with the amino acid sequence SEQ ID No. : 9. 9. In certain embodiments the anti-IL13 antibodies contain a heavy chains with the amino acid sequence SEQ ID No. : 10. In certain embodiments the anti-IL13 antibodies contain a lightchain with the amino acid sequence SEQ ID No. : 14. In certain embodiments the anti-IL13 antibodies include a heavy chain region with the amino acid sequence SEQ ID No. The anti-IL13 antibody may include a heavy chain variable region with the amino acid sequence SEQ ID No. : 9. In certain embodiments the anti-IL13 antibodies comprise a heavy chains having the amino acids sequence of SEQID NO. The anti-IL13 antibody may include a heavy chain with the amino acid sequence SEQ ID No. : 14. In certain embodiments the amount of hamster-PLBL2 can be quantified by an immunoassay, or mass spectrometry. In certain embodiments the immunoassay used is a total Chinese hamster ovary ELISA, or a hamster PLBL2 ELISA. In certain embodiments the mass spectrometry test is LC/MS/MS.
Click here to view the patent on Google Patents.